What and when?
NICE guidance refers to “rescue therapy” as either insulin-based treatment and/or sulfonylurea therapy. In people with type 2 diabetes, it recommends the use of rescue therapy at any phase of treatment if there is symptomatic hyperglycaemia, and reviewing treatment when blood glucose control has been achieved.1
Assessment for osmotic symptoms should be undertaken at diagnosis, especially if HbA1c is markedly raised, and at subsequent reviews, or when therapy intensification is indicated. Symptoms of hyperglycaemia (polydipsia, polyuria, lethargy, skin infections, slow-healing wounds) and weight loss can occur in type 2 diabetes but may also suggest insulin deficiency, for example in type 1 diabetes or pancreatogenic (type 3c) diabetes, for which insulin would be the preferred therapy.2
Temporary insulin therapy may also be required in people with type 2 diabetes during periods of acute illness and admission to hospital, and in those on steroid therapy or chemotherapy, when marked hyperglycaemia is not sufficiently managed with oral or non-insulin injectable glucose-lowering therapies.
The primary aim of rescue therapy is to alleviate symptoms and to ensure safety if the diabetes diagnosis is unclear or if type 1 diabetes is suspected.
Sulfonylurea rescue therapy
Commonly used sulfonylureas are gliclazide and glimepiride. Their glucose-lowering effect is usually seen within a few days of initiation provided there is adequate pancreatic beta-cell function. An HbA1c reduction of 11–22 mmol/mol (1.0–2.0%) can be expected, when added to lifestyle measures.3
Blood glucose monitoring should be offered to assess efficacy, optimise safe dose titration and to prevent/avoid hypoglycaemia. This is especially important in people who drive. Due to the potential risk of hypoglycaemia, it is not safe to titrate the medication without assessing glucose monitoring profiles.
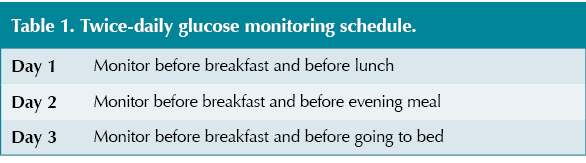
Recommended monitoring advice is to measure glucose levels before meals, ideally monitoring four times daily (before breakfast, before lunch, before the evening meal and before bed). If this is not possible, monitoring twice daily at varied times may be appropriate (see Table 1).
At least weekly contact (telephone or face-to-face consultations) after initiating sulfonylurea therapy is necessary to assess response to treatment and titrate doses based on glucose trends and patterns.
For more information, see Prescribing pearls: A guide to sulfonylureas.4
If there is little or no response to sulfonylurea therapy, this could suggest insulin deficiency and insulin therapy may be required.
Sulfonylurea driving regulations
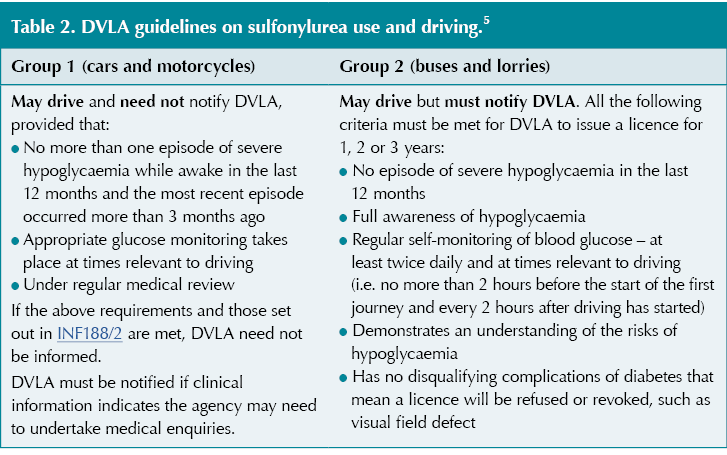
The DVLA recommends that for Group 1 licence holders (cars and motorcycles) treated with sulfonylureas, it is appropriate to offer glucose monitoring at times relevant to driving to enable the detection of hypoglycaemia. For Group 2 licence holders (bus and lorry), regular self-monitoring of blood glucose at least twice daily and at times relevant to driving should be undertaken (see Table 2).5
See How to assess fitness to drive for more information on licensing requirements.6
Insulin rescue therapy
Insulin is the preferred therapy in certain circumstances, specifically if severe hyperglycaemia is present (HbA1c >86 mmol/mol [10.0%]), and especially if associated with weight loss or ketonuria/ketosis, in underweight people of if type 1 diabetes is suspected.3
Where the diabetes diagnosis is unclear and/or type 1 diabetes is suspected, insulin should not be delayed whilst awaiting specialist review or diabetes autoantibody testing and should be started pre-emptively. In this instance, a basal–bolus regimen may be the most appropriate. Whether this is initiated in primary or secondary care will depend on local service provision.
After insulin initiation, hyperglycaemic symptoms often improve rapidly even if ongoing dose adjustment is needed to achieve optimal glucose targets.
NICE recommends people with an HbA1c >75 mmol/mol (9.0%) to start a pre-mixed (biphasic) insulin, although in clinical practice basal insulin is often the initial insulin utilised in many people with type 2 diabetes requiring rescue therapy.
Numerous formulations of insulin are available, with differing time–action profiles and durations. Prescribing guidance will play a role in what is available to prescribe and initiate in different localities. For all available insulins and delivery devices in the UK, see the DSN Forum UK Insulin types and delivery devices comparison chart.7
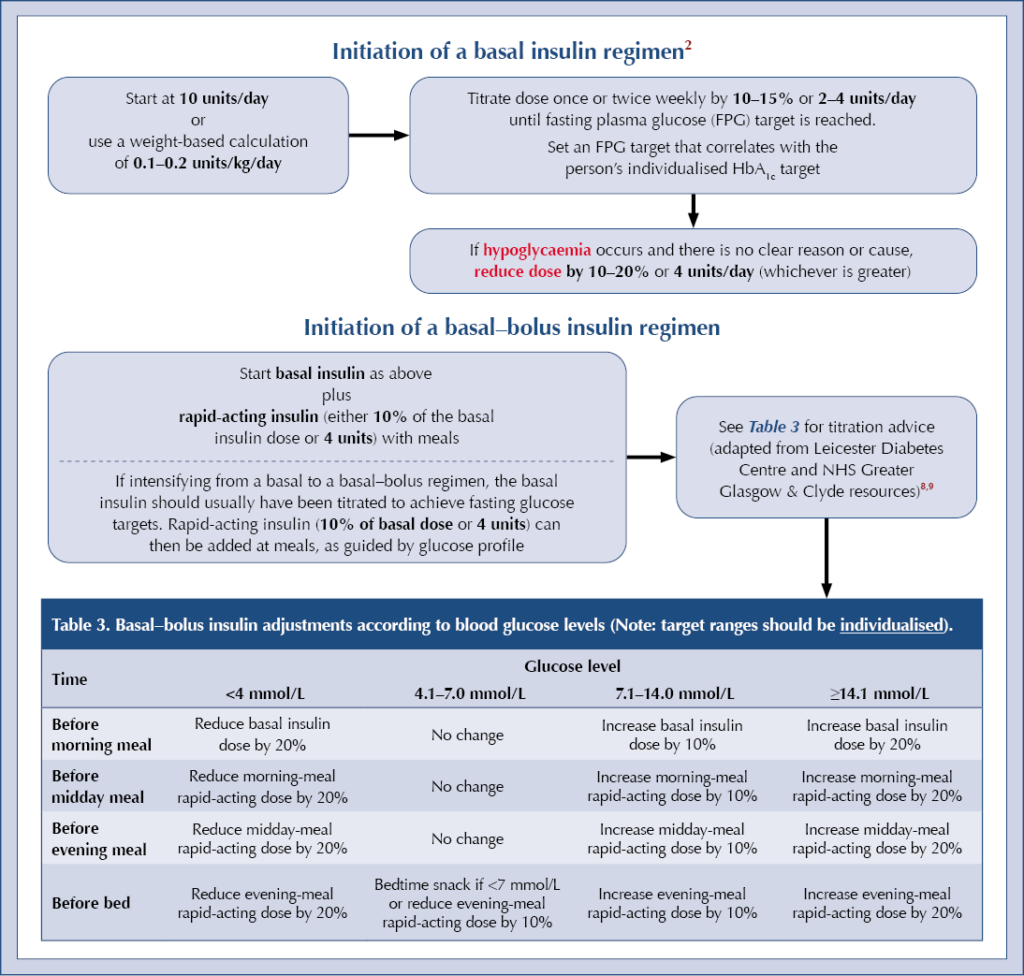
Initiation of a basal insulin regimen2
Insulin driving regulations
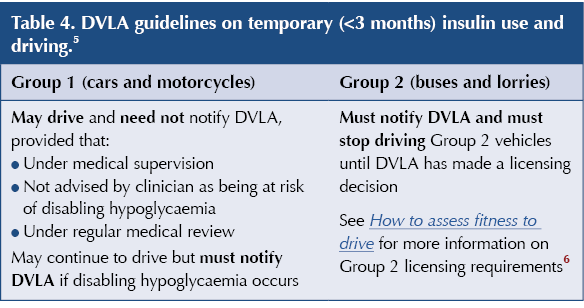
Where insulin therapy continues for less than 3 months, Group 1 drivers may continue to drive as long as the criteria in Table 4 are met. In contrast, drivers of Group 2 vehicles must inform the DVLA and not drive Group 2 vehicles until approved by the DVLA.
By law, people initiated on insulin for more than 3 months must inform the DVLA (and their insurance company) and practise glucose monitoring at times relevant to driving. The requirements are different for Group 1 and Group 2 licensing (see How to assess fitness to drive for more information).6
Deprescribing of rescue therapy
In people with a confirmed diagnosis of type 2 diabetes who require rescue therapy, once their symptomatic hyperglycaemia has resolved it is always advisable to review whether this is the most appropriate glucose-lowering therapy in the longer term. The addition of organ-protective medications (e.g. SGLT2 inhibitors, GLP-1 receptor agonists) should be considered in line with local/national guidance.
The addition of such therapies is likely to result in a need to de-escalate rescue therapy – see case studies below. For further advice, see At a glance factsheet: Deprescribing in type 2 diabetes.10
If type 1 diabetes is suspected and insulin has been initiated, discuss with the specialist team for ongoing management.
Putting theory into practice
These two case studies demonstrate practical advice on how and when to initiate rescue therapy and subsequent de-escalation within a primary care setting.
Case study 1
A 44-year-old South Asian man with a new diagnosis of type 2 diabetes presented with a 3–4-week history of osmotic symptoms and tiredness.
BMI 24 kg/m2. No ketones were present. HbA1c was 123 mmol/mol (13.4%), random blood glucose 24.3 mmol/L. He had not lost any weight and was otherwise well. His mother had type 2 diabetes diagnosed at >50 years old.
Commenced on gliclazide 40 mg twice daily and explained that insulin may be needed based on response to treatment.
Taught how to perform home blood glucose monitoring and advised to measure glucose before meals and before bedtime. Does not drive. Initial glucose levels 18–24 mmol/L. Hypoglycaemia advice given.
Reviewed after 1 week, glucose levels 12–17 mmol/L, osmotic symptoms improving. Gliclazide continued at 40 mg in the morning and increased to 80 mg in the evening.
Reviewed a week later, glucose levels 8–15 mmol/L. Osmotic symptoms completely resolved, therefore started on metformin 500 mg once daily for 1 week, increasing to 500 mg twice daily thereafter.
Reviewed 2 weeks after starting metformin – glucose levels 5–10 mmol/L and tolerating metformin with no side effects. Advised to increase metformin to 1 g with breakfast and 500 mg with evening meal for 1 week, and then to increase to 1 g twice daily. Advised to stop gliclazide.
Reviewed 4 weeks later – capillary glucose levels 4–9 mmol/L. Less frequent monitoring advised, with view to stop. QRISK score >10% so started on SGLT2 inhibitor therapy as per NICE guidance.
HbA1c rechecked 3–4 months later and was 49 mmol/mol (6.6%).
Case study 2
A 35-year-old white man presented with recurrent genital thrush and osmotic symptoms. He had lost weight over the past 2 years (from 20 stone to 17 stone) but attributed this to a change to a vegetarian diet in this time. His BMI was 34 kg/m2. His maternal grandmother had type 2 diabetes but there was no other family history of diabetes. A random blood glucose was 19.1 mmol/L, urinary ketones 1+ and HbA1c 124 mmol/mol (13.5%).
He was seen by the GP and, given the weight loss, presence of ketones (although at low levels), osmotic symptoms and level of hyperglycaemia, agreed to start insulin therapy as the safest course of action until a diagnosis of type 1 diabetes could be excluded. He was taught how to monitor his glucose levels and started on Humulin I, 10 units once daily every morning. He was given a dual glucose and ketone meter and advised to check for ketones when glucose was >15 mmol/L or if unwell. He was advised on how to recognise hypoglycaemia and given advice on how to treat this appropriately.
GP reviewed a week later – fasting glucose levels 16.7–24.6 mmol/L. Humulin I increased to 12 units once daily. Ketone levels all less than 0.6 mmol/L. Advice given on how to self-titrate insulin.
GP reviewed 2 weeks later. On Humulin I, 18 units once daily. Fasting glucose levels 14–15 mmol/L. Ketones normal. Diabetes autoantibodies test requested and referred to secondary care team.
GP reviewed 2 weeks later. On Humulin I, 28 units once daily. Fasting glucose levels 12.6–19.6 mmol/L. Pre-meal (varied times) readings 12–15 mmol/L. Second dose of Humulin I (10 units) added in the evening with evening meal. Other options at this point could have been switching either to a twice-daily pre-mixed insulin regimen or to a basal–bolus regimen.
GP reviewed 1 week later – glucose levels improving with the additional dose of insulin. Advice given regarding self-titration of insulin.
Seen in secondary care, diabetes autoantibodies (GAD/IA2/TnT8) were negative; therefore, diagnosis deemed likely to be type 2 diabetes. At the time of review, he was on Humulin I, 25 units twice daily, with glucose levels in single figures. HbA1c was 54 mmol/mol (7.1%).
Advised to introduce modified-release metformin starting at 500 mg once daily and titrating up to 2 g per day over a 4-week period. Advice was given to reduce Humulin I to 20 units twice daily, and a referral was made to the diabetes specialist nurse to assist with downtitration of insulin based on his glucose profile. The plan was to add additional SGLT2 inhibitor therapy once established on metformin.
Useful resources
● University Hospitals Leicester Insulin therapies: an educational toolkit
● NHS Greater Glasgow & Clyde Diabetes: Insulin initiation and adjustment, patients with type 2 diabetes, primary care. Guidance for Diabetes Specialist Nurses
● DSN Forum UK Insulin types and delivery devices comparison chart
● Prescribing pearls: A guide to sulfonylureas
● How to minimise insulin errors





Risk ratios of 1.25 for autism spectrum disorder and 1.30 for ADHD observed in offspring of mothers with diabetes in pregnancy.
18 Jun 2025