The management of hypertension in diabetes is crucial in reducing the risk of cardiovascular disease (CVD), which is significantly higher than in people without diabetes. Optimisation of hypertension is also central to the management of microvascular complications, notably nephropathy and retinopathy.
This second of two articles on diabetes and hypertension covers lifestyle change and the use of medication to manage hypertension. The importance of a personalised approach, that takes account of factors such as age, comorbidities and social circumstances, is emphasised. The specific cardiorenal advantages of using drugs that block the renin–angiotensin system are discussed, along with the subsequent building of antihypertensive therapy, including situations where comorbidities might direct drug choice. Resistant hypertension and treatment of hypertension in pregnancy are reviewed.
Individualising therapy
The blood pressure (BP) targets and treatments advised in guidelines are, by their nature, generic. It is important to recognise that individual circumstances can modify these goals, a point emphasised in both NICE (2023) and ADA (2024) guidelines. Thus, age, degree of frailty, ethnicity, comorbidities, concurrent medications, life expectancy and social support need to be considered.
The elderly may have impaired hepatic and renal function, leading to reduced metabolism and excretion of drugs and subsequent drug toxicity; polypharmacy can exacerbate these problems. Antihypertensive medication side effects, including falls, electrolyte disturbance and acute kidney injury (AKI), are more likely to occur in the elderly (Cushman et al, 2010; Sink et al, 2018). For these reasons, the NICE standard BP target of <140/90 mmHg for people with diabetes is increased in those aged ≥80 years to <150/90 mmHg, but even higher targets and less intensive antihypertensive therapy may be clinically valid. In contrast, a lower BP target and more intensive treatment may be appropriate for those with albuminuria, retinopathy or at high risk of stroke (<130/80 mmHg if <80 years; NICE, 2023).
A person-centred approach should be adopted, with explanation of the benefits and risks of antihypertensive treatment leading to shared decision-making in which individual preferences are taken into account.
Lifestyle change
There is clear evidence that lifestyle intervention can reduce BP in people with diabetes (Arauz-Pachecho et al, 2002; Chen et al, 2015). An important advantage of lifestyle change is that it is generally not accompanied by significant side effects.
Lifestyle issues should always be reviewed when antihypertensive medication is being considered. Changes should be discussed and agreed with individuals, with the aim that they are achievable and sustainable. Box 1 lists some of the important components of lifestyle advice recommended in guidelines that cover hypertension and diabetes (NICE, 2022a; NICE, 2023; ADA, 2024).
Switching to a healthy diet can lead to a BP reduction equivalent to that of a single antihypertensive drug, with the further benefits of improving glycaemic and lipid levels (DASH, 2024). It has been estimated that a 1 kg loss in body weight is associated with a 1 mmHg reduction in systolic BP (Semlitsch et al, 2016). In addition, weight loss is linked to reduced insulin resistance and improved glycaemic levels in type 2 diabetes.
The DASH (Dietary Approaches to Stop Hypertension) eating programme specifically addresses hypertension and, in individuals without diabetes, was demonstrated to lower BP to a similar extent as a single antihypertensive medication (DASH, 2024). A high-fibre and low-sodium diet appears to have the greatest effect in lowering both systolic and diastolic BP in people with type 2 diabetes (Abbasnezhad et al, 2020). Remember that the psychosocial aspects driving eating (comfort eating, rather than eating to satisfy hunger) may need attention (Nash, 2017).
To address lifestyle issues, offer people with diabetes referral to local diabetes education programmes from the point of diagnosis. The NHS Type 2 Diabetes Path to Remission programme, which is being rolled out across Integrated Care Boards by NHS England, employs a low-calorie diet and lifestyle support to achieve substantial weight loss that could lead to diabetes remission and lowering of BP (Bakhai, 2023).
Obstructive sleep apnoea is an important cause of hypertension, and addressing it has been shown to reduce BP in people with type 2 diabetes (Shaw et al, 2016).
Review medications taken by the individual, as these may have a hypertensive effect. Common examples are non-steroidal anti-inflammatory drugs, corticosteroids and the combined contraceptive pill.

When should antihypertensive medication be offered?
For people with diabetes, NICE recommends discussing starting antihypertensive drug treatment in all people <80 years of age with diabetes who have a sustained clinic BP of ≥140/90 mmHg, or an ambulatory or home BP monitoring (ABPM/HBPM) reading of ≥135/85 mmHg (NICE, 2023). The decision whether to use antihypertensive medication will be influenced by other considerations, including age, frailty and comorbidities. The presence of established CVD or high cardiovascular risk, heart failure, renal disease or target-organ damage will strengthen the case for antihypertensive medication.
If BP is ≥180/120 mmHg and there is evidence of target-organ damage (e.g. retinopathy, albuminuria or reduced eGFR, or left-ventricular hypertrophy on ECG), then consider the immediate introduction of antihypertensive therapy without waiting for ABPM/HBPM results (NICE, 2023; see Part 1).
First-line antihypertensive therapy in people with diabetes
Due to their cardiorenal protective properties, angiotensin-converting enzyme inhibitors (ACEis; e.g. ramipril or lisinopril) or angiotensin receptor blockers (ARBs; e.g. losartan or irbesartan) are recommended as first-line antihypertensive therapy for people with both type 1 (NICE, 2022b) and type 2 diabetes (NICE, 2023). These agents have been demonstrated to reduce cardiovascular events in people with diabetes (Catala-Lopez et al, 2016) and to exert a renoprotective effect over and above their effect on BP. This is particularly evident in individuals with proteinuria (urinary albumin–creatinine ratio [ACR] >30 mg/mmol; Palmer et al, 2015).
The ACEi/ARB benefits extend to normotensive people with proteinuria. Thus, even without hypertension, people with chronic kidney disease (CKD) and diabetes (types 1 or 2) should be offered an ACEi or ARB, titrated to the highest tolerated dose, if ACR is ≥3 mg/mmol to slow the progression of kidney disease (de Boer et al, 2017; NICE, 2021). In the absence of albuminuria, the cardiorenal benefits of ACEis and ARBs are less clear (Bangalore et al, 2016).
Whilst the actions of ACEis and ARBs in providing BP optimisation and cardiorenal protection are considered interchangeable, an ARB is preferred to an ACEi in people with Black African or African–Caribbean family origin because it is less likely to induce cough and angioedema (NICE, 2023).
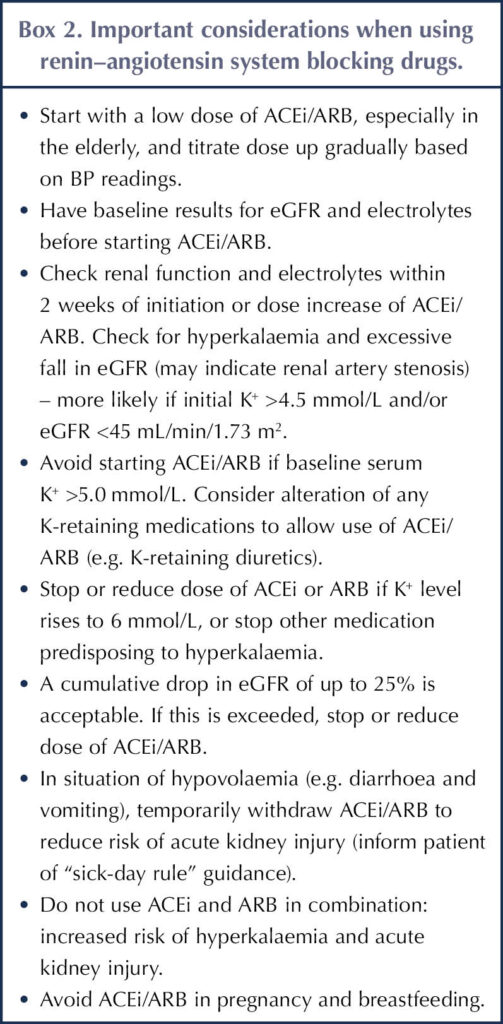
The combination of an ACEi and ARB does not appear to improve outcomes, and increases the risk of AKI and hyperkalaemia – which predispose to cardiovascular events – so is not recommended (Fried et al, 2013; Makani et al, 2013; ADA, 2024). Box 2 outlines some important considerations when using ACEis or ARBs (de Boer et al, 2017; NICE, 2021).
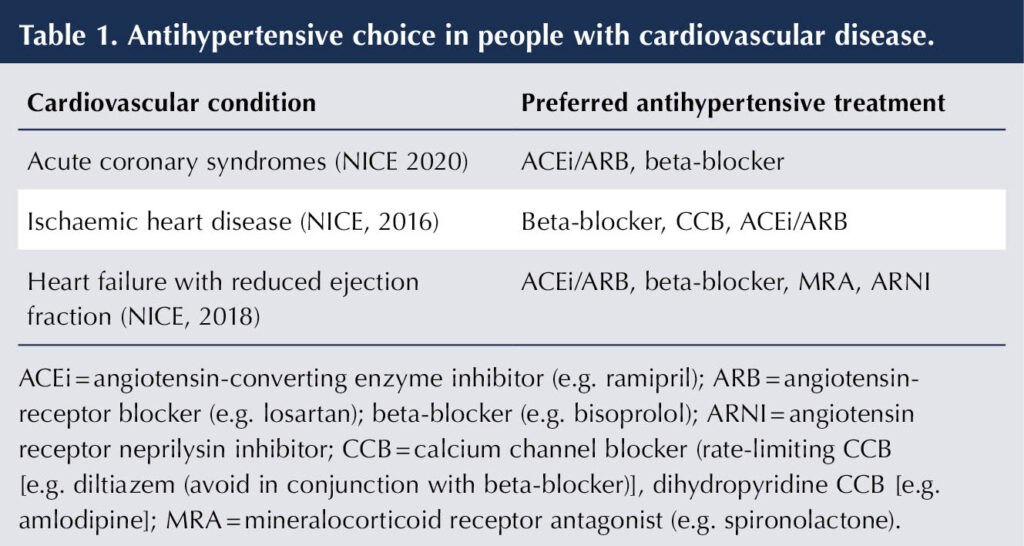
The choice of antihypertensive medication can be influenced by comorbidities. In the situation of coexisting CVD, the specific benefits of certain antihypertensive therapies will promote their use (see Table 1). For many individuals with diabetes, a combination of antihypertensive drugs will be required to reach their BP goal.
Building antihypertensive therapy
The dose of ACEi or ARB should be titrated up to manage hypertension, or to the maximum tolerated dose if there is a specific indication for cardiorenal disease (e.g. heart failure or albuminuric CKD). If the maximum tolerated dose does not achieve the target BP, first check that the individual is taking the treatment as prescribed.
Next, add either a dihydropyridine calcium channel blocker (CCB) or a thiazide-like diuretic (NICE, 2023; ADA, 2024). There is evidence that both these classes of medication improve cardiovascular outcomes in subjects with diabetes (Barzilay et al, 2004; Weber et al, 2010). Comorbid cardiovascular conditions may direct a preference for one or other of these agents, as summarised in Table 1.
If an ACEi or ARB is contraindicated or not tolerated, then a CCB or thiazide-like diuretic treatment would become first-line treatment. Ultimately, the most important goal is to achieve satisfactory BP levels with antihypertensive agents that can be tolerated.
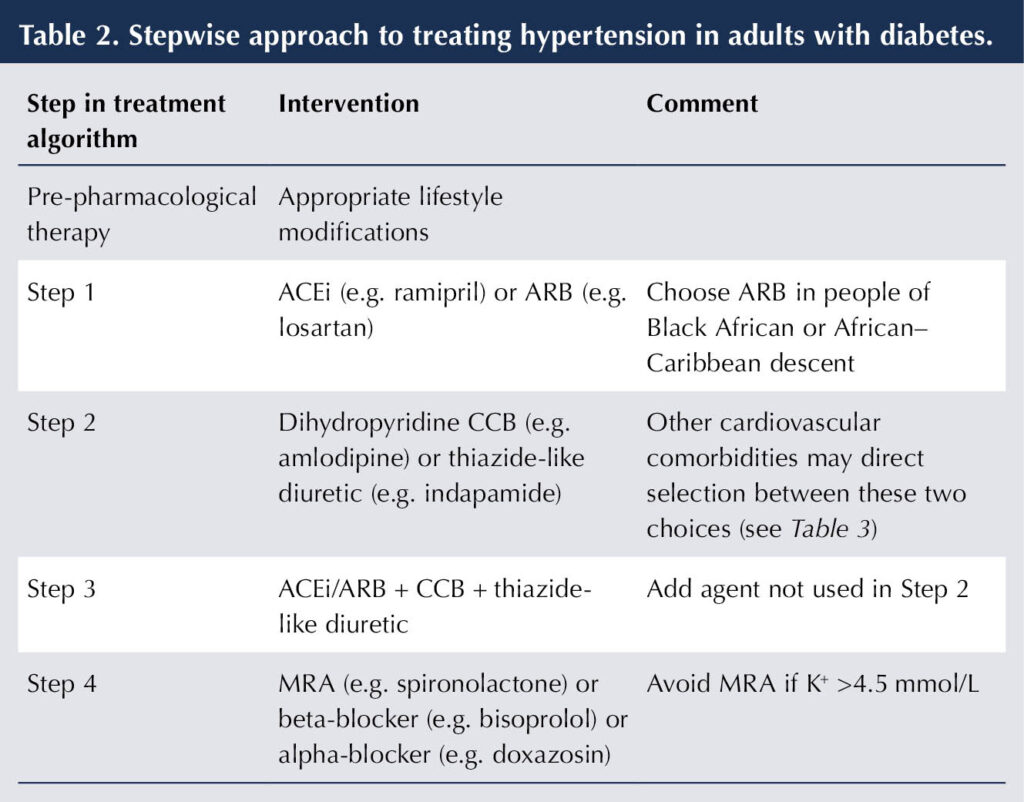
If dual antihypertensive therapy does not achieve target BP, then a third agent should be added, such that triple therapy would consist of ACEi/ARB + CCB + thiazide-like diuretic. The stepwise approach is summarised in Table 2.
It should be noted that thiazide-like diuretics become less effective below an eGFR of 30 mL/min/1.73 m2 and a loop diuretic should be used instead (de Boer et al, 2017).
Resistant hypertension
Blood pressure that is not controlled satisfactorily, despite the use of three antihypertensive therapies, is defined as resistant hypertension (NICE, 2023). In such a situation, ensure that existing medications are being tolerated and taken appropriately. Adherence is less likely the greater the number of antihypertensive medications being prescribed (Sheppard et al, 2022). Check for postural hypotension. Offer ABPM/HBPM to confirm resistant hypertension.
If the diagnosis of resistant hypertension is confirmed, a fourth antihypertensive agent can be added (see Table 2). There is evidence that mineralocorticoid-receptor antagonists (MRAs) are the most effective add-on agent for resistant hypertension when added to the combination of ACEi/ARB + CCB + thiazide-like diuretic (Williams et al, 2015; de Boer et al, 2017). Both NICE (2023) and ADA (2024) recommend their use at this stage.
Low-dose spironolactone is usually chosen but, if gynaecomastia proves problematic, then eplerenone is an alternative. Avoid using an MRA if serum K+ level is >4.5 mmol/L. When they are employed, check for hyperkalaemia within 2 weeks of initiation (more likely if ACEi/ARBs are also being used and in the situation of pre-existing renal failure).
In addition to their role in heart failure, MRAs possess the additional benefit on reducing albuminuria in people with diabetic nephropathy (ADA, 2024). The non-steroidal MRA finerenone is licensed specifically for this indication (BNF, 2024).
If K+ level is >4.5 mmol/L, then alternative options for treating hypertension are an alpha-blocker (e.g. doxazosin) or a beta-blocker (e.g. bisoprolol; NICE, 2023).
Should BP remain above target despite optimal use of four antihypertensive drugs, then specialist advice will be required.
Antihypertensive medication side effects
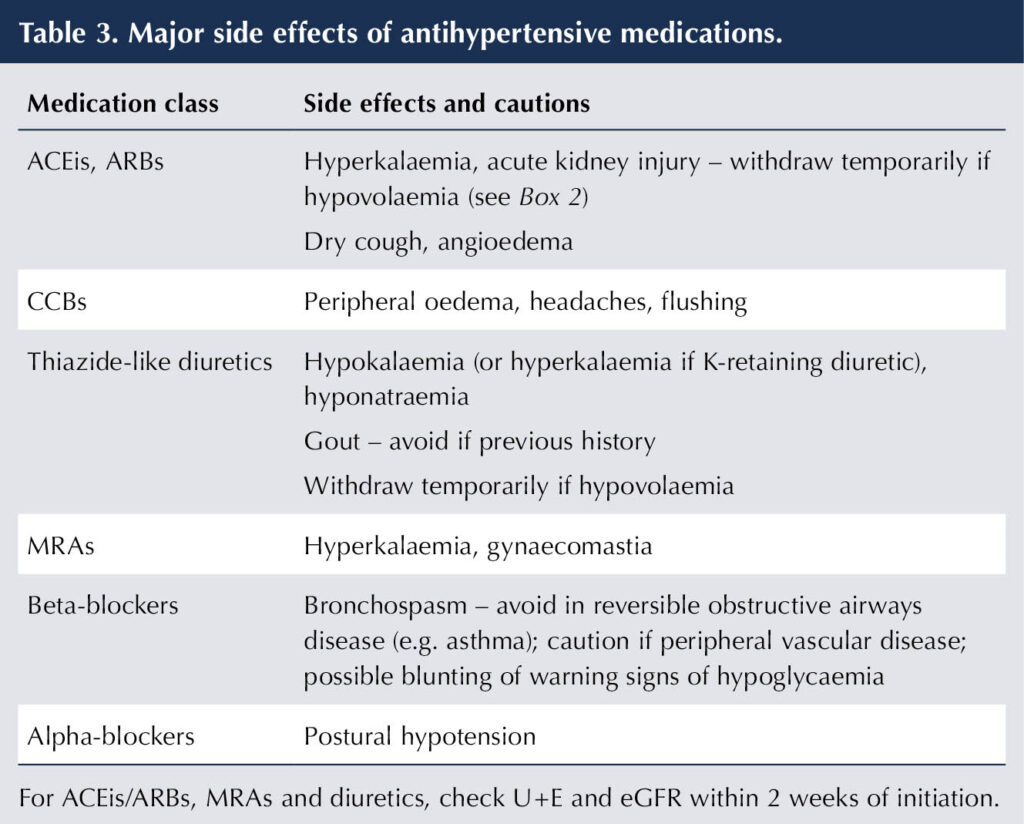
Some important side effects from using antihypertensive medications are listed in Table 3. These may require drug withdrawal or dose reduction, with introduction of an alternative antihypertensive, as necessary.
Blood pressure management and medications for hyperglycaemia
Use of sodium–glucose cotransporter 2 (SGLT2) inhibitors can lead to a small reduction in BP (typically 4/2 mmHg). Glucagon-like peptide-1 (GLP-1) receptor agonists are also associated with a small antihypertensive effect, although this is only around half that of SGLT2 inhibitors (de Boer et al, 2017).
Evidence would suggest that ACEis/ARBs and CCBs may have a slightly favourable effect on glucose control, whereas beta-blockers and diuretics have a slightly adverse effect. However, the magnitude of these changes is very small and outweighed by the beneficial effects of antihypertensive action on cardiovascular outcomes (Ferrannini and Cushman, 2023).
Pregnancy and diabetes
In managing hypertension in pregnancy, first reinforce lifestyle measures – a healthy diet with reduced salt intake, exercise and weight management. NICE recommends offering antihypertensive treatment if BP >140/90 mmHg in pregnancy, aiming for 135/85 mmHg (NICE, 2019) (see details of targets for pre-existing and gestational hypertension in Part 1). The key issue is choosing medications known to be safe in pregnancy.
ACE inhibitors, ARBs and spironolactone are known to be teratogenic and are, therefore, contraindicated in pregnancy. Diuretics should also be avoided, though they may be an option in late-stage pregnancy (ADA, 2024). If a woman with hypertension is planning a pregnancy and is already taking these classes of medication, then they should be discontinued in favour of alternative treatments that are safe to use in pregnancy.
Because of their long track-record of safety, the three antihypertensives most commonly used are labetalol, methyldopa and slow-release nifedipine (NICE, 2019). A Cochrane systematic review concluded that beta-blockers and CCBs were the most effective safe treatments (Abalos et al, 2018). Labetalol is licensed for use in pregnancy and is considered first-line therapy by NICE. If labetalol is not suitable, then nifedipine modified-release may be used (although NICE advise using a brand that is not contraindicated in pregnancy – so check the SmPC). Methyldopa is a further option, though this may be less efficacious than labetalol and nifedipine, and more prone to causing side effects, including nausea, dry mouth, headache, dizziness and drowsiness. Antihypertensives may need to be used in combination to achieve satisfactory BP control.
Women with pre-eclampsia or more severe hypertension may require hospital admission for BP management. After delivery, women with gestational hypertension and pre-eclampsia should have their BP closely monitored and medications adjusted and withdrawn, as necessary. In the longer term, BP and other cardiovascular risk factors should be continued to be monitored because they remain at higher cardiovascular risk (NICE, 2019).
Case history 1.
Presentation
Omari is a 58-year-old man of African ethnicity and a history of type 2 diabetes for 7 years. BP control has always been difficult and, despite attention to lifestyle and use of triple antihypertensive therapy (losartan 100 mg once daily, indapamide 2.5 mg once daily and amlodipine 10 mg once daily), his current clinic BP is 155/86 mmHg.
There is a family history of stroke.
How would you manage Omari’s hypertension?
Outcome
- Check that Omari is tolerating his antihypertensive medications and taking them correctly.
- Take the opportunity to address any lifestyle issues that might impact on BP control.
- Emphasise the importance of good BP control in avoiding complications in diabetes.
Omari has, by definition, resistant hypertension. This is subsequently confirmed using ABPM, which averages 149/81 mmHg. Checking recent blood tests at review, his eGFR is 74 mL/min/1.73 m2, with a K+ level of 4.1 mmol/L.
He is offered spironolactone 25 mg once daily, with arrangements for repeat electrolytes and renal function test in 2 weeks, and a follow-up appointment in one month.
Case history 2.
Presentation
Jean is a 76-year-old lady with type 2 diabetes, taking triple antihypertensive therapy of ramipril 10 mg once daily, lercanidipine 10 mg once daily and bendroflumethiazide 2.5 mg once daily. She has complications of diabetic retinopathy and diabetic nephropathy (stage G3b A2 [eGFR, 43 mL/min/1.73 m2; urinary ACR, 27 mg/mmol]).
Jean attends the surgery because of recurrent episodes of dizziness on standing, with near black-outs. Her clinic BP is 128/55 mmHg, with a significant fall on standing.
How would you manage this situation?
Outcome
- The diagnosis of postural hypotension appears clear.
- Jean’s BP control is too intensive and she is at risk of injury from falling.
- Avoidance of falls is more important than the tight control of BP that might be dictated by her diabetic nephropathy.
Jean’s bendroflumethiazide is discontinued, aiming to avoid volume depletion. Ramipril, which offers renoprotection and cardiovascular benefits, is continued at a lower dose of 5 mg once daily. Jean is advised on avoidance of falls and a review appointment to recheck symptoms and BP is made for 1 month.
Conclusion
Blood pressure targets and treatments in people with diabetes should take account of individual circumstances, including age, frailty, comorbidities, concurrent medications and social circumstances.
Lifestyle factors that impact adversely on hypertension should be addressed, as there is clear evidence that this can result in improvement in BP in people with, and without, diabetes.
ACE inhibitors and ARBs are first-line antihypertensive agents in people with diabetes because of their cardiorenal benefits. An ARB is preferred to an ACEi in people with Black African or African–Caribbean family origin because it is less likely to induce cough and angioedema.
Dihydropyridine CCBs and thiazide-like diuretics are the preferred agents to build antihypertensive therapy in subjects with diabetes, with MRAs effective in resistant hypertension. Beta-blockers and alpha-blockers are further antihypertensive options. The approach to antihypertensive use may be altered by the presence of cardiovascular comorbidities, including ischaemic heart disease, heart failure with preserved ejection fraction, atrial fibrillation and post-myocardial infarction.
Potassium levels and renal function need to be closely monitored when using ACEis/ARBs, MRAs and diuretics, with hyperkalaemia and AKI being the greatest concerns.
For hypertensive women with diabetes planning pregnancy or for hypertension diagnosed in pregnancy, the recommended antihypertensive treatments are labetalol, slow-release nifedipine and methyldopa. These should replace or be chosen in preference to ACEis/ARBs, MRAs and diuretics, which are thought to be teratogenic. Management is likely to take place in an antenatal clinic.





Study provides new clues to why this condition is more aggressive in young children.
14 Nov 2025