Since their introduction to the UK 11 years ago, sodium–glucose cotransporter 2 (SGLT2) inhibitors have become increasingly prominent as a treatment for type 2 diabetes. In addition to glycaemic control, SGLT2 inhibitors offer a useful weight loss and a low risk of hypoglycaemia, and their ease of use and good tolerability has led to extensive use in primary care. In recent years, SGLT2 inhibitors have been shown to improve outcomes in people with type 2 diabetes with or at high risk of cardiovascular (CV) disease. Beneficial effects in heart failure (HF) and chronic kidney disease (CKD) from use of SGLT2 inhibitors have been demonstrated, with these benefits extending outside the type 2 diabetes population.
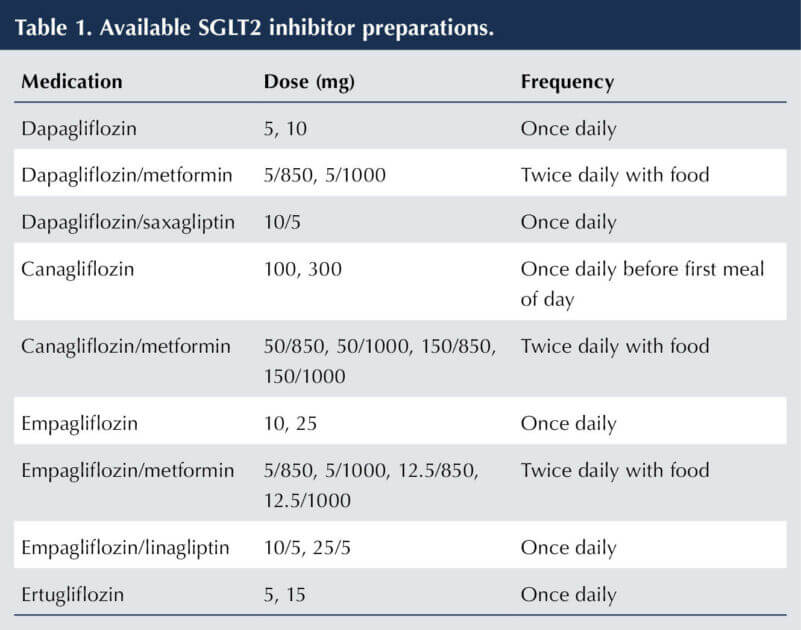
Four SGLT2 inhibitors are licensed for use in the UK: canagliflozin, dapagliflozin, empagliflozin and ertugliflozin. Combination preparations with metformin or DPP-4 inhibitors are available (see Table 1; Morris 2022; BNF, 2023).
This article focuses on the cardiorenal benefits of the SGLT2 inhibitors and the translation of these benefits into clinical practice.
Utility for glycaemic control in type 2 diabetes
Typically, HbA1c reductions of around 7–11 mmol/mol (0.6%–1.0%) are obtained from using SGLT2 inhibitors in type 2 diabetes, with larger reductions anticipated with higher starting HbA1c values (Xu et al, 2022). The improvement in glycaemic control seen with SGLT2 inhibitors is principally achieved through selective inhibition of glucose resorption at the SGLT2 transporter site in the proximal convoluted tubule of the kidney, leading to urinary glucose excretion.
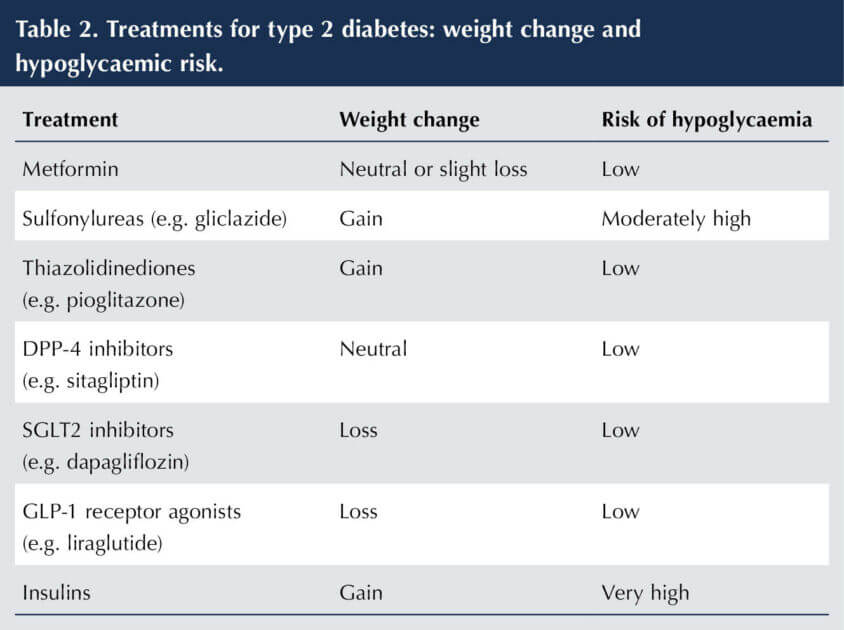
Metformin remains the first-line pharmacological treatment for glycaemic control in type 2 diabetes, following dietary and lifestyle advice. When a second agent is required, NICE advises choosing between sulfonylureas, pioglitazone, DPP-4 inhibitors and SGLT2 inhibitors. This choice will depend on the patient characteristics and requirements (NICE, 2022a). Thus, SGLT2 inhibitors are a compelling choice where weight loss and/or avoidance of hypoglycaemia are priorities (see Table 2). This is likely to be the case in the majority of people with type 2 diabetes, and has been a driving force for the rapid expansion of SGLT2 inhibitor use.
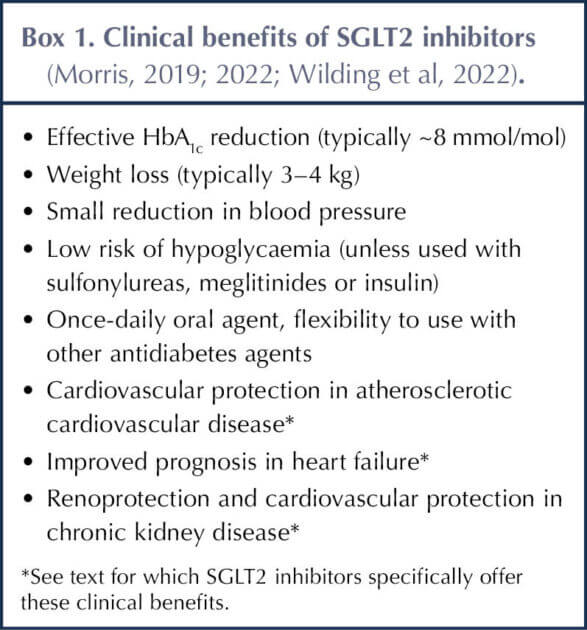
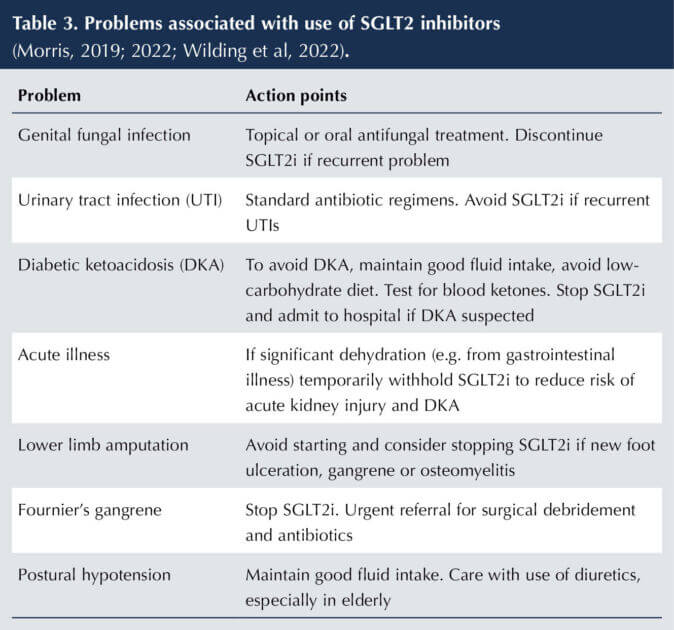
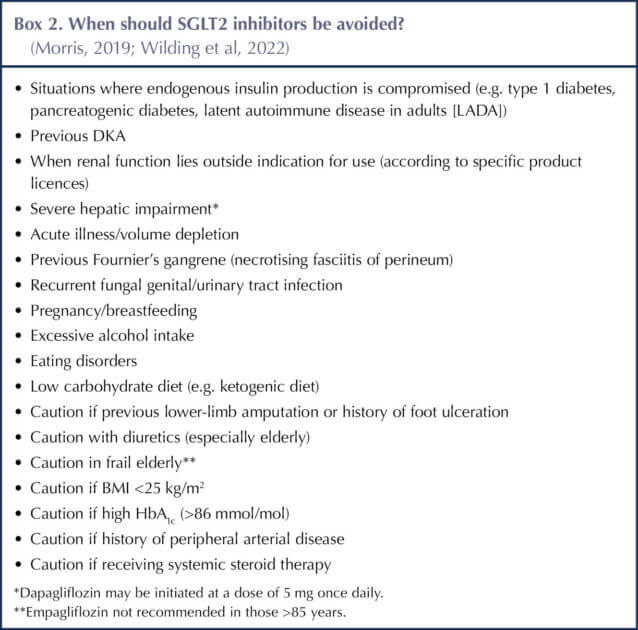
The clinical benefits and drawbacks of SGLT2 inhibitors are summarised in Box 1 and Table 3, and have been elaborated previously in this journal (Morris, 2019; 2022). Situations in which SGLT2 inhibitors should be avoided are listed in Box 2.
If metformin is contraindicated or not tolerated (including trying the modified-release preparation to reduce the risk of gastrointestinal disturbance), then any of the four treatments mentioned above, including SGLT2 inhibitors, are options as monotherapy for glycaemic control (NICE, 2016a; NICE, 2019; NICE, 2022a)
Atherosclerotic cardiovascular disease: special considerations for SGLT2 inhibitors
The evidence
The randomised, double-blind, placebo-controlled trials EMPA-REG OUTCOME (Zinman et al, 2015) and CANVAS (Neal et al, 2017), with empagliflozin and canagliflozin respectively, established that these treatments are effective in improving CV outcomes (defined by MACE – major adverse cardiovascular events – a composite of CV death, non-fatal myocardial infarction and non-fatal stroke) in subjects with type 2 diabetes and established ASCVD (i.e. secondary prevention).
The CV outcome trial (CVOT) with dapagliflozin (DECLARE-TIMI 58), which included two thirds of participants without established atherosclerotic CV disease (ASCVD) (i.e. largely a primary prevention cohort), showed a non-significant reduction in MACE versus placebo, but did find a significant reduction in the composite co-primary outcome of CV death and hospitalisation for HF (Viviott et al, 2019). The CVOT with ertugliflozin (VERTIS) in a type 2 diabetes population with established ASCVD found CV safety, but not superiority, over placebo (Cannon, et al 2020).
NICE guidance and practical implications
These findings have clear consequences for the person with type 2 diabetes who has, or is at high risk of, ASCVD and have led to an alteration in NICE guidance with regard to prescribing of SGLT2 inhibitors (NICE, 2022a).
For the newly diagnosed person with type 2 diabetes who has existing ASCVD – coronary heart disease, including angina and myocardial infarction; stroke or transient ischaemic attack; or peripheral vascular disease – (secondary prevention) the following course of action is recommended:
- Offer metformin plus an SGLT2 inhibitor with proven CV benefit (empagliflozin, canagliflozin, dapagliflozin) as initial dual therapy.
- Metformin should be commenced first and, once tolerability is established, the SGLT2 inhibitor with proven CV benefit can be added without re-measuring HbA1c.
- If metformin is contraindicated or not tolerated, then use the SGLT2 inhibitor alone.
Should, at any point, a person with type 2 diabetes develop ASCVD, you should:
- Start an SGLT2 inhibitor with proven CV benefit, if this is not already being taken – either adding to, or substituting for, existing treatment.
For the person with type 2 diabetes at high risk of CV disease (primary prevention), consider:
- Adding in (or substituting) an SGLT2 inhibitor with known CV benefit (canagliflozin, empagliflozin, dapagliflozin).
- High risk is defined as having a QRISK CV risk score of >10% for the next 10 years in a person of ≥40 years (preferably using the QRISK3 calculator [QRISK, 2023], although, if IT systems have not yet had this embedded, then the less comprehensive QRISK2 calculator should be used) or in the case of someone <40 years having an associated CV risk factor (hypertension, dyslipidaemia, obesity, smoking, first-degree relative with early CVD).
Use of SGLT2 inhibitors in heart failure: a seismic change
The evidence
The CVOTs with the SGLT2 inhibitors consistently showed benefits for people with HF as a secondary outcome. Subsequently, dedicated randomised controlled trials with HF as a primary outcome have been performed.
Note that HF can be associated with three ranges of left-ventricular ejection fraction (LVEF).
- HF with reduced ejection fraction (HFrEF) with LVEF of ≤40%. Termed “systolic HF”, with impaired left-ventricular contraction. Approximately 50% of cases of HF (Murphy et al, 2020).
- HF with mildly reduced ejection fraction (HFmrEF), with LVEF of 41%–49%.
- HF with preserved ejection fraction (HFpEF), with LVEF of ≥50%. Termed “diastolic HF”, where the problem is impaired left-ventricular relaxation rather than left-ventricular contraction.
Both the DAPA-HF (McMurray et al, 2019) and EMEROR-Reduced (Packer et al, 2020) trials, with dapagliflozin and empagliflozin respectively, demonstrated a reduction in the composite outcome of worsening HF and CV death compared to placebo, largely driven by the reduction in HF, in a population with HFrEF (i.e. LVEF ≤40%). Significantly, the treatment groups in these trials were already receiving largely optimised medication for HF (ACE inhibitors, angiotensin receptor blockers [ARBs], beta-blockers, mineralocorticoid receptor antagonists [MRAs] or diuretics). Strikingly, the benefits occurred whether or not the person had type 2 diabetes and persisted at lower eGFR.
An improvement in outcomes for HFmrEF or HFpEF (i.e. LVEF >40%) have also been demonstrated for empagliflozin (EMPEROR-Preserved trial) and dapagliflozin (DELIVER trial) versus placebo (Anker et al, 2021; Solomon et al, 2022). As with HFrEF, the benefits for HFpEF/HFmrEF occurred irrespective of whether type 2 diabetes was present or not.
NICE guidance and practical implications
Based on this evidence, an SGLT2 inhibitor (dapagliflozin 10 mg once daily or empagliflozin 10 mg once daily) should be offered to all people with symptomatic HF (HFrEF or HFpEF), whether or not they have type 2 diabetes, unless contraindicated (Pop-Bussi et al, 2022). This is now reflected in their licensing (dapagliflozin [emc, 2023a]; empagliflozin [2023b]). For people with HFrEF and HFmrEF, NICE recommends that the SGLT2 inhibitor should be used as an add-on to optimise standard care with (NICE, 2021a; 2021b; 2022b; 2023):
- ACEi or ARB + beta-blocker + (if tolerated) MRA, or
- Sacubitril/valsartan may be offered instead of an ACEi or ARB to improve symptoms (this treatment should only be initiated in the context of a specialist HF service).
Although there are no specific trials of these “standard care” medications in HFmrEF, analysis of trials that included people with HFmrEF did indicate that their benefits also extended to this population (NICE, 2023).
In the situation of HF in people with type 2 diabetes, the SGLT2 inhibitor could be used alongside metformin at the point of diagnosis of type 2 diabetes – as described above for atherosclerotic heart disease – or if HF is diagnosed at any point subsequently (NICE, 2022a). The initiation of an SGLT2 inhibitor may well take place in the context of a specialist HF clinic, but could reasonably be undertaken in primary care where there is experience with the use of SGLT2 inhibitors and management of HF.
Diastolic HF (HFpEF) will typically be diagnosed and managed in secondary care. SGLT2 inhibitors are a welcome addition to the treatment options as, currently, only loop diuretics to relieve fluid overload are advised as a pharmacological intervention in HFpEF (other than general measures to reduce risk factors – antiplatelet, statin, antihypertensive medications; NICE, 2023).
Renoprotection and cardiovascular protection from SGLT2 inhibitors in chronic kidney disease
The two components of CKD are reduced eGFR and albuminuria. In the context of diabetes, diabetic nephropathy is a likely precipitant of CKD, though there may be additional causes, notably hypertension.
The evidence
In the CVOTs of empagliflozin, canagliflozin and dapagliflozin, significant benefit was demonstrated in a secondary composite renal endpoint compared to placebo (Wanner et al, 2016; Neal et al, 2017; Wiviott et al, 2019), whilst in the ertugliflozin CVOT the advantage over placebo showed a strong trend that just fell short of significance (Cannon et al, 2020).
Following these trials, randomised controlled trials have been performed comparing the SGLT2 inhibitors versus placebo with a primary renal outcome. Thus, the CREDENCE trial showed canagliflozin to be superior to placebo in individuals with type 2 diabetes who had albuminuria and a range of renal function using a primary composite endpoint composed of end-stage renal disease, doubling of serum creatinine and death from renal or CV cause (Perkovic et al, 2019).
This was soon followed by the DAPA-CKD trial, which again demonstrated a reduced rate of progression of CKD and decreased CV mortality for dapagliflozin versus placebo. Crucially, these benefits were seen whether or not the individuals had type 2 diabetes (Heerspink et al, 2020) and whatever their baseline renal function. More recently, similar positive results for deterioration of renal function and death from a CV cause were confirmed for empagliflozin in the EMPA-KIDNEY trial, again regardless of whether people had type 2 diabetes or not (Herrington et al, 2023).
Initially, SGLT2 inhibitors cause a reduction in eGFR of 4–6 mL/min/1.73 m2 owing to their effect in reducing intraglomerular pressure – a haemodynamic effect that is reversible. Subsequently, renal function is stabilised, with a slower rate of decline and a 30%–50% reduction in albuminuria.
NICE guidance and practical implications
Canagliflozin and dapagliflozin are now licensed for use in CKD in people with type 2 diabetes (alongside other therapies, such as ACEis and ARBs; emc, 2022; 2023a). Dapagliflozin is further indicated for CKD in the absence of type 2 diabetes when albuminuria is present. Empagliflozin awaits licensing for CKD (with or without type 2 diabetes).
In line with NICE guidance, for people with type 2 diabetes and CKD, in addition to treatment with an ARB or ACEi titrated to the maximum licensed tolerated dose, you should (NICE, 2021c; 2022a):
- Offer an SGLT2 inhibitor if urinary albumin–creatinine ratio (uACR) is >30 mg/mmol.
- Consider an SGLT2 inhibitor when uACR is between 3 and 30 mg/mmol.
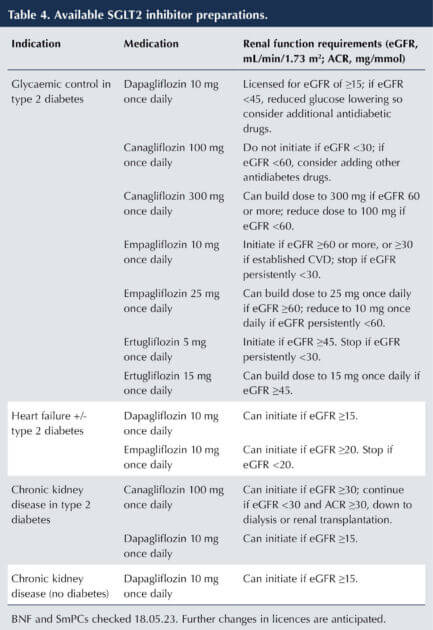
- Prescribe dapagliflozin and canagliflozin in line with their licensed eGFR and uACR specifications (see Table 4).
Furthermore, dapagliflozin is recommended by NICE as a treatment for CKD in the absence of type 2 diabetes in adults taking an optimised dose of ARB or ACEi (NICE, 2022c), unless these are contraindicated, if:
- eGFR lies in the range 25–75 mL/min/1.73 m2 at the start of treatment; and
- uACR is ≥22.6 mg/mmol.
The recommended values of eGFR and ACR for use of dapagliflozin in people without type 2 diabetes relate to the parameters of the population enrolled in the DAPA-CKD trial. For people with type 2 diabetes, there is additional evidence of renal benefit from use of dapagliflozin in populations with eGFR down to 15 mL/min/1.73 m2 and lower uACR values from DECLARE-TIMI 58 and DAPA-HF trials (NICE, 2022c).
Prescribing tips
The widening indications for use of SGLT2 inhibitors and the renal function requirements for starting and withdrawing treatment for those indications are summarised in Table 4 (emc, 2022; 2023a; 2023b; 2023c; BNF, 2023; Brown, 2023).
It is important to note that whilst the glucose-lowering potential of the SGLT2 inhibitors falls in parallel with declining renal function, their renoprotective properties are maintained, as is their effectiveness in HF. Thus, the SmPCs of SGLT2 inhibitors state that glucose-lowering efficacy will fall in moderate renal failure (eGFR <45 mL/min/1.73 m2) and will likely be absent in severe renal impairment, such that additional glucose-lowering treatments should be considered.
How do SGLT2 inhibitors produce cardiorenal benefits?
The mechanisms of action underpinning the CV and renal protection seen with SGLT2 inhibitors remain uncertain. Glycaemic lowering cannot account for these benefits, since they are not found with other antidiabetes medications that produce an equivalent glucose-lowering effect. The trial evidence (see above) clearly demonstrates that the benefits in HF and CKD extend equally to the non-diabetes population.
Conventional risk factors that might contribute to improved cardiorenal outcomes include blood pressure lowering (typically 3–5 mmHg systolic, 1–2 mmHg diastolic) and body weight reduction (typically 3 kg).
A more subtle explanation suggests SGLT2 inhibitors improve myocardial and renal energetics by shifting hormonal balance toward glucagon that favours free fatty acid and ketone metabolism. Increased levels of beta-hydroxybutyrate (a ketone) provide a more efficient energy substrate for the heart and kidneys (Ferrannini et al, 2016).
The natriuresis and the glycosuria-mediated osmotic diuresis caused by the SGLT2 inhibitors improve prognosis in HF by reducing plasma volume that leads to removal of interstitial fluid, thus reducing pulmonary congestion and oedema (Heerspink et al, 2018).
A plausible mechanism of action explaining the renoprotection offered by SGLT2 inhibitors is that of vasoconstriction of the afferent glomerular arteriole that results in reduction of intraglomerular pressure, protecting the glomerular basement membrane and reducing albuminuria (Anders, 2016). By inhibiting sodium resorption, the SGLT2 inhibitor facilitates sodium delivery to the macula densa in the distal tubule, and subsequent tubuloglomerular feedback leads to afferent arteriolar vasoconstriction. Given that ACEis and ARBs exert their renoprotective effect via vasodilation of the efferent arteriole of the glomerulus, then the expectation would be that the renal benefits of the SGLT2 inhibitors would be additive to these agents.
Other proposed mechanisms that could account for the cardiorenal benefits of SGLT2 inhibitors include (Zelniker and Braunwald, 2020):
- Reduced visceral adiposity resulting in a fall in adipose tissue-mediated cytokine production and a decreased inflammatory effect – reducing atherosclerosis, and benefiting HF and renal function.
- Reduced accumulation of advanced glycation end-products and, hence, less risk of fibrosis affecting cardiac and renal function.
- Reduction in oxidative stress, an important factor in the development of ischaemic heart disease, possibly via a beneficial effect on mitochondrial function.
- Stimulation of renal erythropoietin secretion leading to an increased haematocrit that could favour heart and kidney metabolism through improved oxygen delivery.
- Triggering loss of uric acid by secretion into the tubules. Uric acid is associated with increased inflammation and oxidative stress.
Conclusion
SGLT2 inhibitors have moved to the centre stage of pharmacological treatment of type 2 diabetes in primary care. This transformation has occurred because of the multiple benefits offered by SGLT2 inhibitors and their ease of use. In addition to facilitating improvement in glycaemic control with a low risk of hypoglycaemia and weight loss, the SGLT2 inhibitors offer benefits to people with, or at high risk of, atherosclerotic heart disease, HF and CKD. Strikingly, cardiorenal benefits extend to those without type 2 diabetes, further increasing the scope of use for these agents.






Charity publishes its 10-year vision to transform diabetes prevention, care and treatment.
22 May 2025