This article updates a previous review on sodium–glucose cotransporter 2 (SGLT2) inhibitors in this journal (Morris, 2019) by discussing evidence from recent trials, the impact it has had on licensed indications and guidelines, and how this can be translated into clinical practice.
Introduction
SGLT2 inhibitors lower blood glucose levels via selective inhibition of glucose resorption at the SGLT2 transporter site in the proximal convoluted tubule of the nephron, facilitating excretion of glucose in the urine. Further details of this process have been described in this journal previously (Morris, 2017).
NICE, SIGN and American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) guidelines have all positioned SGLT2 inhibitors as an option for second-line therapy (after metformin and including dietary/lifestyle intervention) to achieve adequate glycaemic control (NICE, 2015a; 2016; 2019; SIGN, 2017; Buse et al, 2020). These and other guidelines have stressed the importance of an individualised HbA1c target and assessing therapeutic choice based on the individual’s comorbidities and needs.
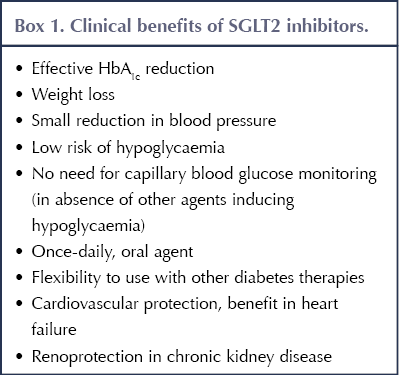
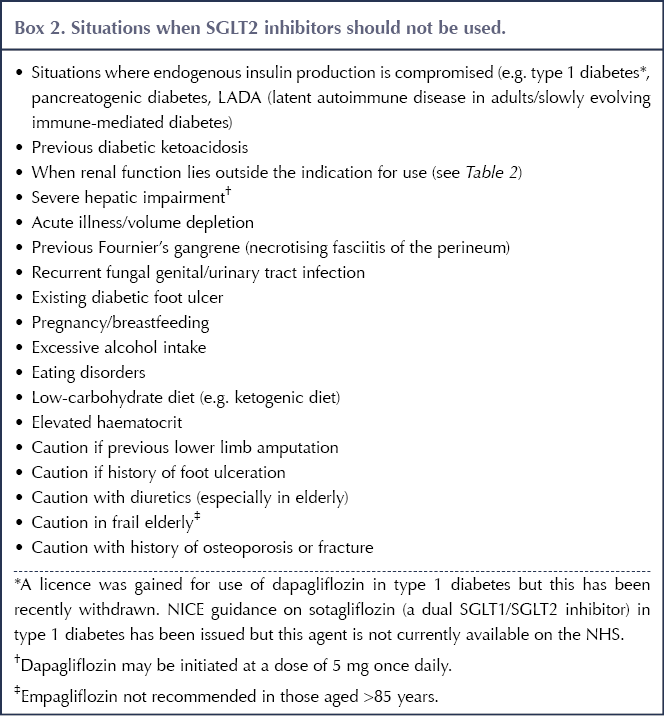
Originally developed as glucose-lowering agents for type 2 diabetes that could facilitate weight loss and did not cause hypoglycaemia, SGLT2 inhibitors have since demonstrated additional advantages in the management of people with atherosclerotic cardiovascular disease (ASCVD), heart failure (HF) and chronic kidney disease (CKD). The benefits offered by SGLT2 inhibitors are summarised in Box 1, while the situations where they should be avoided or used with caution are outlined in Box 2 (Morris, 2019).
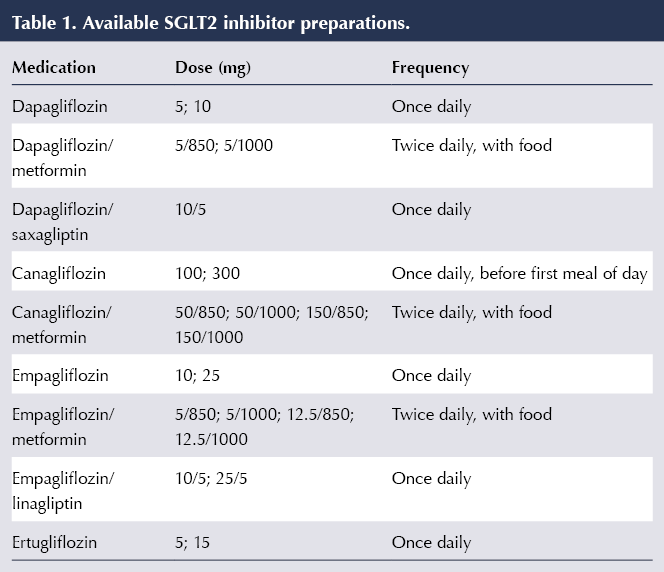
At present, four drugs within the SGLT2 inhibitor class are licensed to treat type 2 diabetes in the UK. These, along with preparations in which the drugs are combined with metformin or DPP-4 inhibitors, are listed in Table 1.
Recent outcome trials
1. Cardiovascular outcome trials (CVOTs)
The ground-breaking EMPA-REG OUTCOME study, reported in 2015, provided convincing evidence for secondary prevention of cardiovascular events in people with type 2 diabetes. In this randomised, double-blind, placebo-controlled trial, empagliflozin demonstrated a significant reduction in the primary cardiovascular (CV) outcome of 3-point MACE (major adverse cardiovascular events) – a composite of CV death, non-fatal myocardial infarction and non-fatal stroke – versus placebo (Zinman et al, 2015). This result was mirrored in the canagliflozin CVOTs (combined data from the CANVAS and CANVAS-R studies), reported in 2017 (Neal et al, 2017).
The CVOT of dapagliflozin, DECLARE-TIMI 58, reported in 2018, recruited a population with type 2 diabetes in which two thirds of participants did not have established CVD (and so was predominantly a primary prevention cohort). The dual primary outcomes for the trial were MACE and the composite outcome of CV death and hospitalisation for HF (HHF). Although the trend towards a reduction in MACE with dapagliflozin versus placebo was non-significant, a significant reduction in the composite outcome of CV death and HHF was observed, driven principally by a 27% relative risk reduction in HHF (Wiviott et al, 2019). This reduction occurred whether or not there was pre-existing ASCVD or HF.
More recently, the VERTIS CV study randomised a population with type 2 diabetes and established ASCVD to ertugliflozin or placebo, with a primary outcome of 3-point MACE. Whilst CV safety was established, ertugliflozin did not demonstrate superiority over placebo for the primary outcome (Cannon et al, 2020).
Implications for practice
For people with type 2 diabetes who have established ASCVD (angina, myocardial infarction, transient ischaemic attack, stroke or peripheral vascular disease), the cardiovascular protection offered by SGLT2 inhibitors (specifically empagliflozin and canagliflozin) makes them one of the agents of choice (alongside GLP-1 receptor agonists) to improve glycaemic control following lifestyle management and metformin treatment (Buse et al, 2020). While there is evidence for primary prevention of CVD with SGLT2 inhibitors, this is less robust than the evidence for secondary prevention.
2. Heart failure studies
Improvement in HF outcomes was a consistent finding in the CVOTs of the SGLT2 inhibitors, although these were generally secondary outcomes (with the exception of the dapagliflozin trial, in which one of the primary outcomes included HHF as a composite outcome).
DAPA-HF (McMurray et al, 2019) was a randomised controlled trial comparing dapagliflozin versus placebo in individuals with heart failure with reduced ejection fraction (HFrEF), either with or without type 2 diabetes (45% and 55% of participants, respectively). The primary outcome was a composite of CV death and worsening HF (defined by hospitalisation or an urgent visit requiring intravenous therapy for HF). Compared with placebo, individuals receiving dapagliflozin had a 4.9% absolute risk reduction in the primary outcome over a median of 18 months, equating to a 26% relative risk reduction. These benefits were irrespective of participants’ diabetes status and whether or not baseline eGFR was above or below 60 mL/min/1.73 m2. Importantly, the treatment group was already receiving largely optimised medication for HF (ACE inhibitors, ARBs, beta-blockers, MRAs and diuretics).
The findings in the DAPA-HF trial were reflected in the EMPEROR-Reduced study, which demonstrated a reduction in the primary outcome of HHF/CV death with empagliflozin versus placebo in people with HFrEF, with or without diabetes. Again, the positive outcome was largely driven by a reduction in HHF (Packer et al, 2020).
The EMPEROR-Preserved trial investigated the effect of empagliflozin in individuals with heart failure with preserved ejection fraction (HFpEF, or diastolic HF). Compared with placebo, empagliflozin significantly reduced the composite primary outcome of HHF and CV death, irrespective of diabetes status (Anker et al, 2021).
The mechanism of action underpinning the cardiovascular protection seen with SGLT2 inhibitors is uncertain. Conventional risk factors that might contribute include blood pressure lowering and body weight reduction with lower visceral adiposity. A more subtle explanation suggests improved myocardial energetics subsequent to a swing in hormonal balance towards glucagon that increases levels of beta-hydroxy butyrate (a ketone), which is a more efficient energy substrate for the heart (Ferrannini et al, 2016).
Implications for practice
Dapagliflozin and empagliflozin can be used, in addition to standard HF treatment, to prevent worsening of HF and CV death in individuals with type 2 diabetes who have or are at high risk of HF. Both agents are also licensed for treatment of HFrEF in people without type 2 diabetes. The expectation is that the licence for empagliflozin will soon be extended to treat HFpEF.
3. The evidence for renoprotection
CKD may manifest as reduced eGFR and/or albuminuria. Diabetic nephropathy is a likely cause of CKD if other microvascular complications, notably diabetic retinopathy, are present; however, in the absence of these (and in people without diabetes), an alternative cause of CKD should be sought.
Secondary composite renal outcomes in the CVOTs of empagliflozin, canagliflozin and dapagliflozin all demonstrated significant benefits compared with placebo (Wanner et al, 2016; Neal et al, 2017; Wiviott et al, 2019), whilst in the ertugliflozin CVOT the advantage over placebo showed a strong trend that fell just short of significance (Cannon et al, 2020).
Subsequently, studies with dedicated primary renal outcomes have been published. CREDENCE was a double-blind, randomised controlled trial comparing canagliflozin and placebo in people with type 2 diabetes and albuminuric diabetic renal disease, with an eGFR range of 30–90 mL/min/1.73 m2. Results showed a significant 30% relative risk reduction in favour of canagliflozin for the primary composite renal outcome of end-stage renal disease, doubling of serum creatinine, or death from renal or CV causes (Perkovic et al, 2019).
This was soon followed by the DAPA-CKD trial (Heerspink et al, 2020), which again demonstrated an impressive 39% relative reduction in the primary renal outcome for dapagliflozin versus placebo (an absolute risk reduction of 5.3% over a median of 2.4 years; number needed to treat, 19) in albuminuric individuals with a range of renal function (down to eGFR 25 mL/min/1.73 m2). Crucially, similar benefits were seen whether or not the individuals had type 2 diabetes and whatever their baseline renal function.
A plausible mechanism of action explaining the renoprotection offered by SGLT2 inhibitors is that of vasoconstriction of the afferent glomerular arteriole, which leads to a reduction in intraglomerular pressure, thereby protecting the glomerular basement membrane and reducing albuminuria (Anders et al, 2016). Given that ACE inhibitors and ARBs exert their renoprotective effects via the efferent arteriole of the glomerulus, the expectation is that the renal benefits of SGLT2 inhibitors would be additive to these agents.
Implications for practice
Canagliflozin and dapagliflozin offer renal protection and are indicated for the treatment of CKD (alongside other therapies such as ACE inhibitors and ARBs) in people with type 2 diabetes. The renoprotective properties of dapagliflozin extend to those without type 2 diabetes, and dapagliflozin is licensed for use in non-diabetic CKD. It is important to note that whilst the glycaemic-lowering potential of the SGLT2 inhibitors falls in parallel with declining renal function (and, thus, an additional glucose-lowering drug should be considered), their renoprotective properties are maintained.
4. Real-world studies
The observational CVD-REAL study, which retrospectively evaluated over 300 000 people with type 2 diabetes, found that SGLT2 inhibitors were associated with a 39% lower risk of HF and a 51% lower risk of all-cause mortality compared with alternative oral glucose-lowering drugs, whether individuals had established CVD or not (Kosiborod et al, 2017). Although causality cannot be proved in an observational study, the results nevertheless strongly support the benefits of SGLT2 inhibitor use in the real world, away from the restrictions of a randomised controlled trial.
The CVD-REAL 2 study found that SGLT2 inhibitor use conferred a lower risk of death, HHF, myocardial infarction and stroke compared with other glucose-lowering drugs in a multinational study of individuals with type 2 diabetes, of whom just 27% had established CVD (Kosiborod et al, 2018).
Implications for practice
These studies provide reassuring real-world evidence of the CV benefits of SGLT2 inhibitors (compared to use of other diabetes medications) in a widespread population with type 2 diabetes and a broad range of CV risk.
Turning evidence into practice
1. Expansion of licensing
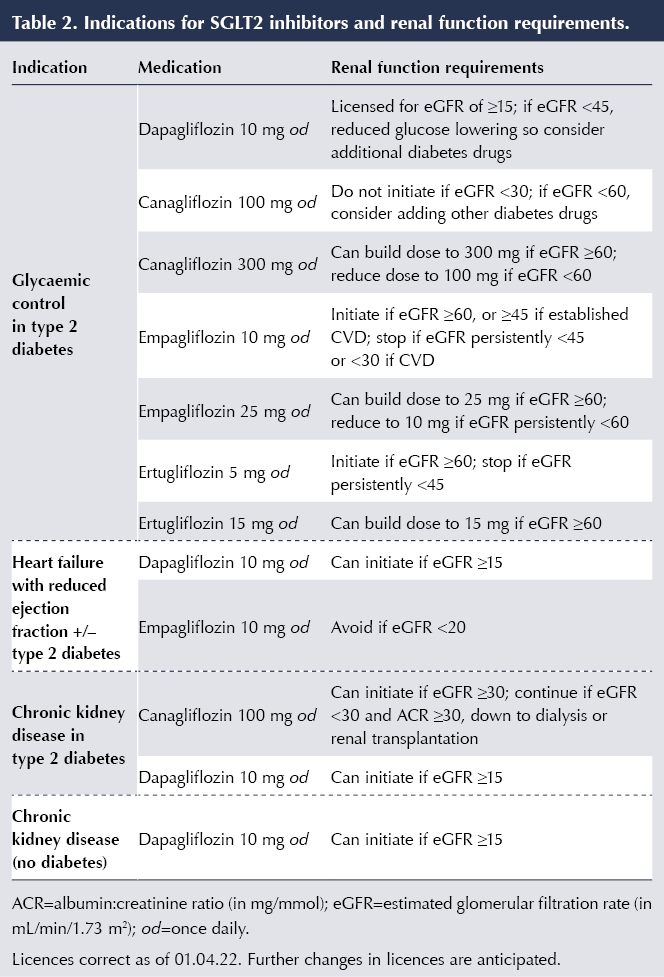
The widening indications for use of SGLT2 inhibitors, and the renal function requirements for starting and withdrawing treatment for those indications, are listed in Table 2.
2. ADA guideline changes
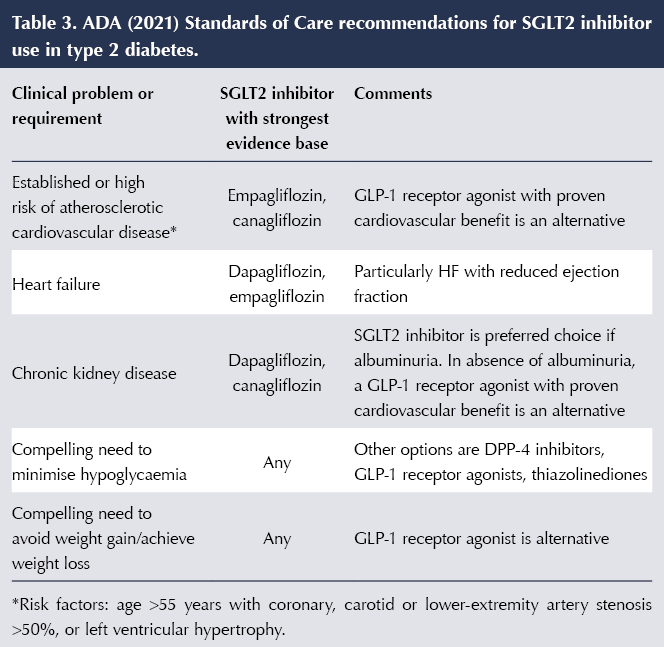
The ADA 2021 Standards of Care further refined the position of SGLT2 inhibitors in light of the latest evidence. This approach is outlined in Table 3 (ADA, 2021). Importantly, in addition to prioritising SGLT2 inhibitors for glycaemic control in the situations listed, the ADA advises consideration of their use in people with ASCVD, HF or CKD independently of baseline HbA1c, individualised HbA1c target or use of metformin.
3. Updated NICE guidelines
The recent update to the NICE (2015a) guideline on management of type 2 diabetes recommends dual first-line therapy with metformin and an SGLT2 inhibitor with proven CV benefit in people with type 2 diabetes who have chronic HF or established ASCVD (myocardial infarction, angina, ischaemic stroke/transient ischaemic attack or peripheral arterial disease). Should any of these situations arise de novo at any point, an SGLT2 inhibitor should be added or switched in for another treatment.
NICE also recommends consideration of this dual first-line therapy for people who have a high risk of CVD (QRISK2 score >10% in people over 40 years of age with type 2 diabetes). Should a person develop this risk of CVD, adding in (or switching to) an SGLT2 inhibitor should be considered.
The advice from NICE is to start with metformin and titrate the dose whilst monitoring for gastrointestinal side effects (switching to the modified-release preparation if necessary). Once the metformin dose is established, the SGLT2 inhibitor can be added (without need for a further HbA1c measurement).
Specific recommendations on the use of dapagliflozin and empagliflozin for HFrEF, as an addition to standard HF treatment, have been made in the TA679 and TA773 technology appraisals (NICE, 2021a; 2021b).
The NICE guideline recommends offering an SGLT2 inhibitor licensed for use in those with type 2 diabetes and CKD (who should already be taking the highest tolerated dose of ACE inhibitor or ARB for renoprotection) if the albumin:creatinine ratio (ACR) is >30 mg/mmol, provided eGFR is within the licensed range for the SGLT2 inhibitor. Consideration of an SGLT2 inhibitor is also recommended if ACR is 3–30 mg/mmol.
It is also worth remembering that CKD is a high-CV-risk condition itself and so, even if the other requirements for use are not met, an individual with CKD could be considered for an SGLT2 inhibitor in order to reduce CV risk.
NICE’s TA775 technology appraisal provides further detail on the use of dapagliflozin as an add-on to ACE inhibitor/ARB therapy in CKD (NICE, 2022).
4. Changes in practice
An important impact of recent evidence and guideline changes is that even if glycaemic control is adequate (in terms of HbA1c), SGLT2 inhibitors with appropriate clinical evidence should be considered in people with ASCVD, HF or CKD. The benefits offered by the agents appear to occur independently of glucose lowering and irrespective of HbA1c at initiation, which is why these benefits extend to individuals without diabetes.
In contrast to when using SGLT2 inhibitors for glycaemic control, the benefits in HF and CKD persist at lower eGFR and, thus, the renal thresholds (eGFR) for initiation and withdrawal of SGLT2 inhibitors for these indications are lower. Thus, dapagliflozin can be used down to an eGFR of 15 mL/min/1.73 m2 both in HFrEF and CKD; canagliflozin can be used down to end-stage renal failure for albuminuric diabetic kidney disease; and empagliflozin can be used down to an eGFR of 20 mL/min/1.73 m2 in HFrEF (see Table 2). However, consider use of an additional glucose-lowering agent if improved glycaemic control is needed in those with an eGFR of <45 mL/min/1.73 m2.
It is clear that SGLT2 inhibitors will find increasing use in the population with type 2 diabetes, even in those whose glycaemic control is satisfactory. They have the strongest evidence of benefit in HF and CKD within the portfolio of medications for type 2 diabetes, and the presence of these comorbidities should trigger consideration of an SGLT2 inhibitor.
In contrast to the ADA/EASD, NICE has not advanced the case for using GLP-1 receptor agonists in people with type 2 diabetes and ASCVD in the NG28 guideline, and this stance is further likely to increase prescribing of SGLT2 inhibitors. However, for this indication (ASCVD), the current licence of SGLT2 inhibitors does not extend to those who do not require improvement in glycaemic control. Therefore, if SGLT2 inhibitors are to be used to treat ASCVD in people with type 2 diabetes with adequate glycaemic control, this would be an off-label use, which would need to be discussed with the individual concerned. (Sequential) co-initiation with metformin, as discussed in the NICE guideline, should be considered.
Using SGLT2 inhibitors in people without type 2 diabetes
The licensed use of dapagliflozin in HFrEF extends to individuals without type 2 diabetes, who derived similar benefit as those with type 2 diabetes in the DAPA-HF trial. Similarly, empagliflozin can be used for treating HFrEF in people with or without type 2 diabetes, and a licence change to include people with HFpEF is expected in the future following results of the EMPEROR-Preserved trial. Results from the DELIVER trial – the use of dapagliflozin in HFpEF – are awaited.
Dapagliflozin is licensed for use in CKD in people without diabetes, in whom it was just as effective as within the diabetes population (Wheeler et al, 2021), again down to an eGFR of 15 mL/min/1.73 m2.
Using SGLT inhibitors in people with type 1 diabetes
In December 2021, the licence of dapagliflozin 5 mg for use in people with type 1 diabetes and a BMI >27 kg/m2 to support glucose control and weight was withdrawn by the manufacturer (MHRA, 2021). This was not due to new safety concerns but rather to avoid “confusion” over the use of dapagliflozin for other indications.
Whether market authorisation will be sought for other SGLT inhibitors remains to be seen. The decision to use SGLT inhibitors in type 1 diabetes will need to be carefully judged. Glycaemic and weight benefits seem clear but are modest and will need to be balanced against the risk of diabetic ketoacidosis (DKA), a potentially fatal complication. Initiation should only be under the supervision of a diabetes specialist (EMA, 2020). It remains uncertain as to whether the cardiorenal benefits of SGLT2 inhibitors seen in people with type 2 diabetes will be replicated in type 1 diabetes.
Issues when using SGLT2 inhibitors
1. Genital fungal infections and urinary tract infections
The glycosuria induced by SGLT2 inhibitors predisposes to genital fungal infection and, to a much lesser extent, urinary tract infection (UTI), with females affected consistently more than males (Unnikrishnan et al, 2018). The strongest predictors of genital fungal infection are female sex and history of previous infection (Thong et al, 2018).
People commencing SGLT2 inhibitors need to be warned about the symptoms of candidal vulvovaginitis and balanitis and that these are relatively common side effects (especially early in treatment) but they respond well to conventional treatments and do not usually require discontinuation of the SGLT2 inhibitor.
Advice on maintenance of good genital hygiene may help prevent occurrence/recurrence of the problem. First-line treatment is with topical antifungal creams and pessaries (e.g. clotrimazole, miconazole). An alternative would be oral fluconazole. If there is marked inflammation, a combination of the antifungal cream with 1% hydrocortisone can help settle symptoms more effectively. Recurrent cases may force discontinuation of the SGLT2 inhibitor, although there is the option of using once-weekly fluconazole 150 mg prophylactically for 6 months (Pappas et al, 2016).
UTIs will usually be treated successfully with increased fluids and standard antibiotic regimens. SGLT2 inhibitors may be better avoided in a person with a history of recurrent UTI. If repeated UTIs develop after commencing SGLT2 inhibitors then discontinuation would be appropriate.
2. Increased urination and volume depletion
The glucosuria induced by SGLT2 inhibitors generates an osmotic diuresis, leading to more frequent voiding that can be troublesome in a minority of users. There may be associated volume depletion side effects, notably postural hypotension, and cautious use in the elderly using diuretics is advised.
3. Acute illness
The glucoretic activity of SGLT2 inhibitors will aggravate the situation of hypovolaemia arising in acute illness (e.g. due to vomiting and/or diarrhoea), and under these circumstances SGLT2 inhibitors should be temporarily discontinued to reduce the risk of acute kidney injury and DKA (Fralick et al, 2017; Wilding et al, 2018). Other medications that should be temporarily stopped include metformin, ACE inhibitors, ARBs and diuretics; however, importantly, insulin should be continued even if not eating. Further sick day rules include ensuring good fluid intake (aim for 3 litres per day), maintaining carbohydrate intake (eat little and often) and seeking medical advice when appropriate (Garg et al, 2018; Down, 2020).
4. Risk of diabetic ketoacidosis
Situations that can predispose to DKA in people taking SGLT2 inhibitors, including acute illness, are listed in Box 3 (Morris, 2019). Typically, DKA occurs when there is a relative deficiency of insulin (and excess of glucagon) that activates lipolysis of free fatty acids and hepatic ketogenesis (Musso et al, 2020). An important feature of DKA linked to SGLT2 inhibitor use is that it may occur at relatively modest levels of blood glucose (<14 mmol/L): so-called euglycaemic DKA (MHRA, 2016; Wilding et al, 2018). A recent systematic review and meta-analysis confirmed the increased risk of DKA with SGLT2 inhibitors compared to placebo or other diabetes drugs (relative risk, 2.13), with an absolute rate of three events per 1000 person-years (Liu et al, 2020).
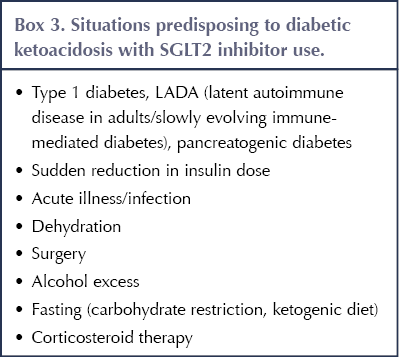
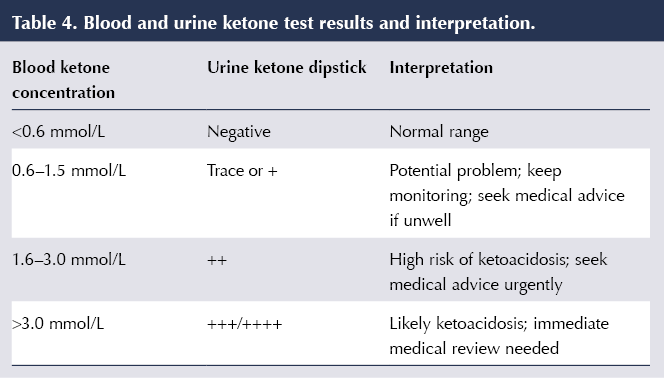
People using SGLT2 inhibitors need to be aware of the warning symptoms of DKA (nausea, vomiting, abdominal pain, shortness of breath, thirst, increased urination, malaise, fruity smell on the breath); when DKA is more likely to occur (even when blood glucose levels are not particularly high); and the need to stop SGLT2 inhibitor therapy and seek immediate medical help should DKA be suspected (MHRA, 2016). Testing for urinary or, preferably, blood (fingerprick) ketones should be performed; interpretation of the results is outlined in Table 4 (Morris, 2019).
Crucially, it is important to educate people starting SGLT2 inhibitors on how to minimise the risk of developing DKA (MHRA, 2016) – see Box 4 (Morris, 2019).

5. Lower limb amputation
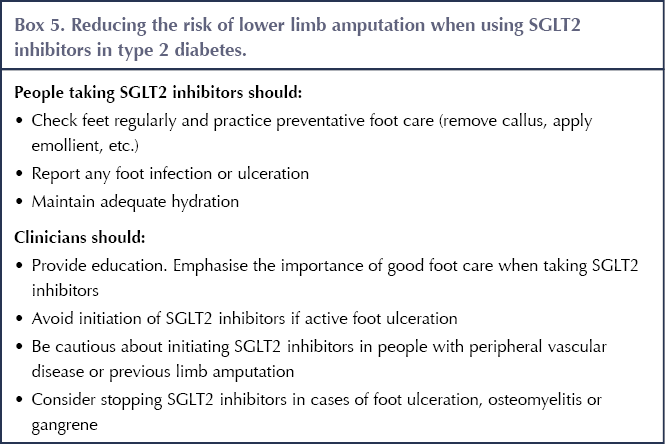
In the CANVAS programme, an increased risk of lower limb amputation was observed with canagliflozin compared with placebo (6.3 vs 3.4 events per 1000 person-years), with the majority of cases involving the toe or metatarsal (Neal et al, 2017). Whilst this was not a significant finding in the dapagliflozin, empagliflozin and ertugliflozin CVOTs, the EMA advised that a warning with regard to lower limb amputation should be included in the Product Information for all SGLT2 inhibitor class members (EMA, 2017).
The MHRA (2017) has provided advice on the risk of lower limb amputation when using SGLT2 inhibitors against the background of foot checks and care advocated by NICE (2015b). Box 5 summarises the approach to minimising the risk of amputation whilst taking SGLT2 inhibitors.
6. Bone fractures
In the canagliflozin CVOT (Neal et al, 2017), a 26% increased risk of fracture compared with placebo was observed, along with reduced bone mineral density at the hip (but not other sites). However, similar findings have not been identified with the other SGLT2 inhibitors. One hypothesis is that a greater incidence of falls secondary to postural hypotension arising from the volume-depleting effects of canagliflozin might have accounted for the increased fracture risk.
Subsequent studies have been reassuring, with a recent meta-analysis not detecting any increased risk of fracture in people with type 2 diabetes treated with SGLT2 inhibitors (Li et al, 2019). Osteoporotic bone fracture is, however, a condition that develops over an extended period of time; therefore, further long-term study is needed to reach definite conclusions on the matter (Wilding et al, 2018).
7. Fournier’s gangrene
Fournier’s gangrene is a potentially life-threatening necrotic soft-tissue infection of the perineum, external genitalia and perianal region, and has been linked to use of SGLT2 inhibitors. Prescribers should be aware of this complication and, if suspected, the SGLT2 inhibitor should be stopped immediately and urgent urological referral made for surgical debridement and treatment with broad-spectrum antibiotics (Bersoff-Matcha et al, 2019).
Conclusions
The SGLT2 inhibitors are established as a key therapy for glycaemic control in people with type 2 diabetes, with the advantageous properties of enabling weight loss and conferring a low risk of hypoglycaemia. Recent evidence has established their importance in the management of heart failure and chronic kidney disease (whether or not type 2 diabetes is present), and their benefit in reducing cardiovascular risk, irrespective of the need for glycaemic control.
Individuals using SGLT2 inhibitors should be aware of the common side effect of genital fungal infection and the rarer, but more serious, possibility of diabetic ketoacidosis. Dehydration from acute illness and new-onset foot ulceration would be reasons to pause treatment/discontinue temporarily, with the plan to restart when dehydration resolves or ulceration clears.
With expanding indications, the therapeutic role of SGLT2 inhibitors looks set to increase. It is essential that healthcare professionals involved in the care of people with diabetes, heart failure and chronic kidney disease keep abreast of developments in these areas.





Helping homeless adults to overcome the challenges of managing their condition.
16 Apr 2024