This article reviews the benefits and problems associated with the use of sodium–glucose cotransporter 2 (SGLT2) inhibitors and their place in the management of type 2 diabetes, and is an update on a previous review in this Journal (Morris, 2017). Recent evidence has clarified and strengthened the position of SGLT2 inhibitor use in type 2 diabetes, and there are also emerging data to support the utility of this class of drug in type 1 diabetes, under restricted and supervised conditions.
How do SGLT2 inhibitors work?
The SGLT2 inhibitors prevent the resorption of glucose that has been filtered from the renal glomeruli. They achieve this by selectively inhibiting glucose transport at the SGLT2 transporter site. As a consequence, glucose is lost in the urine to the extent of 60–80 g per day. Further details on the mechanism of action are described in Morris (2017).
Licensed uses
Four SGLT2 inhibitors are currently available in the UK: dapagliflozin, canagliflozin, empagliflozin and, most recently, ertugliflozin. The licenses have a broad remit, allowing the use of the class in type 2 diabetes as monotherapy or in combination with other glucose-lowering agents, including insulin, when diet and exercise (and other treatments) do not provide adequate glycaemic control.
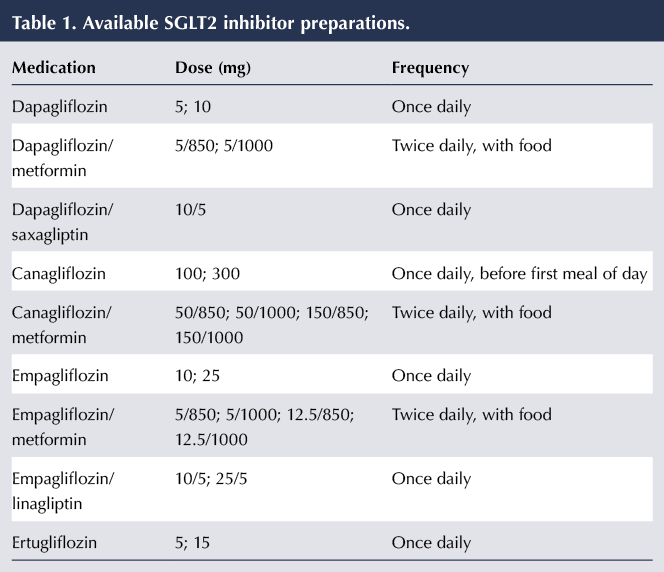
The dose regimens of the SGLT2 inhibitors, including their availability as fixed-dose combinations with other agents, are shown in Table 1.
When should SGLT2 inhibitors be avoided?
There are a number of situations when SGLT2 inhibitors should be used with caution or avoided (Wilding et al, 2018). These are outlined in Box 1.
An important restriction in the licensed use is the instruction not to initiate SGLT2 inhibitors if estimated glomerular function (eGFR) is <60 mL/min/1.73 m2. Once initiated, however, the SGLT2 inhibitors may be continued down to an eGFR of 45 mL/min/1.73 m2 before discontinuation (the lower doses of canagliflozin, empagliflozin and ertugliflozin should be used if eGFR falls below 60). With this in mind, renal function should be checked at least annually, and more frequently if eGFR is <60 mL/min/1.73 m2.
Benefits of SGLT2 inhibitors
The SGLT2 inhibitors are effective in improving glycaemic control either as monotherapy or in combination with other antidiabetes agents, consistently reducing HbA1c by around 6–11 mmol/mol (0.5–1.0%) in clinical trials, and with greater reductions seen in individuals with higher baseline levels of HbA1c (SIGN, 2017; Davies et al, 2018; Marshall, 2018). Weight reduction (typically 2–3 kg) and a small reduction in blood pressure (typically 4/2 mmHg) are also associated with SGLT2 inhibitor use: both desirable outcomes in type 2 diabetes.
An important safety feature of the class is that they do not in themselves increase the risk of hypoglycaemia. Hypoglycaemia is only problematic in the context of an individual taking other diabetes medications that predispose to hypoglycaemia, such as insulin, sulfonylureas and meglitinides; in these situations, it might be prudent to reduce the dose of the latter medications on an individual basis. It is the author’s practice to halve the dose of sulfonylurea or meglitinide, and to cut the insulin dose by around 20% unless the starting HbA1c is particularly high.
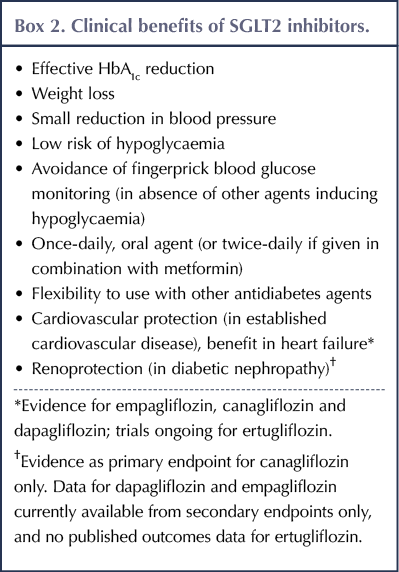
A list of the clinical benefits of SGLT2 inhibitors is summarised in Box 2. Of note are the cardiovascular and renal benefits, the evidence of are discussed in detail below.
Cardiovascular benefits
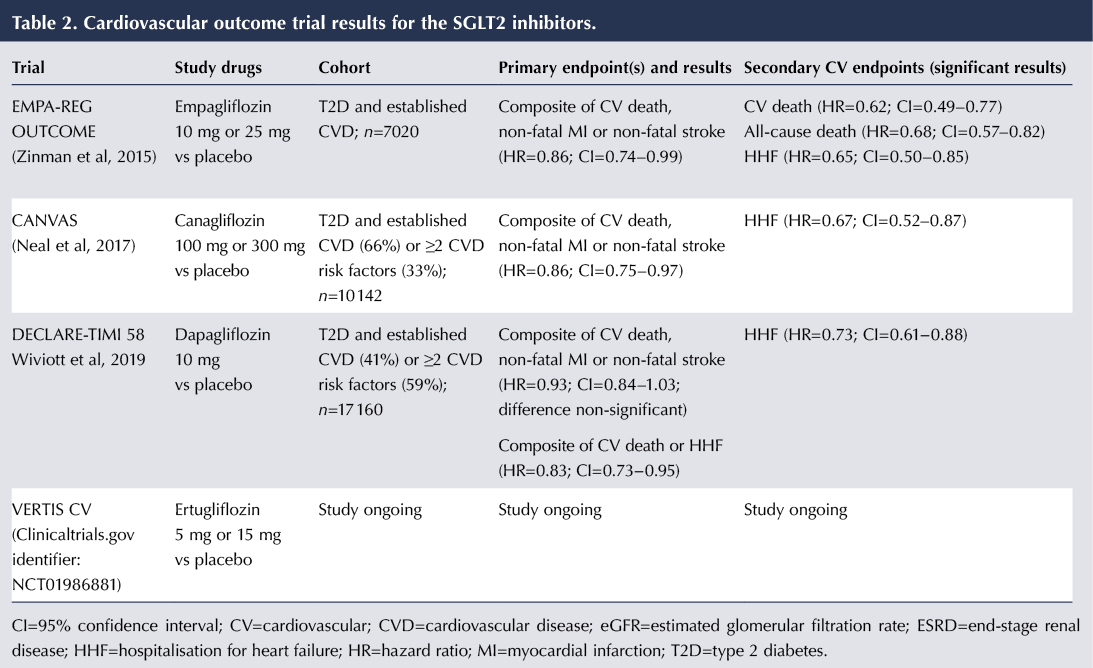
There is now convincing evidence from the cardiovascular outcome trials (CVOTs) of empagliflozin, canagliflozin and dapagliflozin that SGLT2 inhibitors can have a cardioprotective effect in people with type 2 diabetes. A summary of the cardiovascular findings from the CVOTs for empagliflozin, canagliflozin and dapagliflozin is presented in Table 2.
The first evidence of cardiovascular benefit came in 2015 from the landmark EMPA-REG OUTCOME study (Zinman et al, 2015). For the primary outcome, comprising a composite of death from cardiovascular causes, non-fatal myocardial infarction or non-fatal stroke, there was a significant 14% relative risk reduction (RRR) with empagliflozin versus placebo (an absolute risk reduction of 1.6%). This was followed in 2017 by the CANVAS trial, which again showed a 14% RRR in the primary outcome with canagliflozin (Neal et al, 2017).
DECLARE-TIMI 58 (Wiviott et al, 2019) was notable in that two thirds of its participants did not have established cardiovascular disease – it was predominantly a primary prevention cohort rather than a secondary prevention cohort as seen in the empagliflozin and canagliflozin CVOTs. Whilst there was a trend towards a reduced risk of the primary outcome with dapagliflozin, this did not reach significance. There was, however, a reduction in the composite outcome of cardiovascular death and hospitalisation for heart failure (HHF), driven largely by a 27% RRR in HHF, which occurred regardless of history of CVD or heart failure.
The CVOT for ertugliflozin is expected to be reported at the end of 2019.
A further trial worthy of mention is CVD-REAL, an observational study of over 300 000 people with type 2 diabetes across five countries, half of whom received an SGLT2 inhibitor (mainly dapagliflozin or canagliflozin) and half an alternative oral antidiabetes drug (Kosiborod et al, 2017). Only a minority of individuals in the study had established CVD. SGLT2 inhibitors were associated with a 39% lower risk of heart failure and a 51% lower risk of all-cause mortality. Benefits were seen in both people with established CVD and those without. Whilst the study was observational in nature, and therefore causality cannot be proved, it has the merit of being a large study in the “real world”, outside the confines of a clinical trial.
So what can we learn from these studies? In individuals with established CVD, the SGLT2 inhibitors empagliflozin and canagliflozin appear to provide cardioprotection. In relation to heart failure, SGLT2 inhibitors as a class appear to confer benefit, both in those with established CVD and, in the case of dapagliflozin, in those without prior CVD or heart failure.
The mechanism behind the cardiovascular protection seen with SGLT2 inhibitors remains uncertain. Various hypotheses include blood pressure reduction secondary to osmotic diuresis and reduced vascular resistance; body weight reduction leading to lower visceral adiposity; and improved myocardial energetics following an alteration of hormonal balance in favour of glucagon that increases levels of beta-hydroxybutyrate (a ketone), a more efficient fuel for the heart (Ferrannini et al, 2016).
The SGLT2 inhibitors and renoprotection
Whilst a small fall of 3–4 mL/min/1.73 m2 in eGFR (reversible on discontinuation) occurs on commencing SGLT2 inhibitors, it is clear that, over the longer term, renal function stabilises and falls less than with placebo, and the progression of albuminuria is slowed (Wanner et al, 2016; Cherney et al, 2017; Neal et al, 2017; Marshall, 2018; Wiviott et al, 2019).
The CVOTs for empagliflozin, canagliflozin and dapagliflozin all included a composite renal endpoint as a secondary outcome (comprised variously of progression of albuminuria, reduction in eGFR/doubling of serum creatinine, the need for dialysis or death from a renal cause). In all three trials, substantial reductions in the composite renal outcomes were seen in people with type 2 diabetes (including those without established cardiovascular disease), consistent with a renoprotective effect (Wanner et al, 2016; Neal et al, 2017; Wiviott et al, 2019). The EMPA-KIDNEY and DAPA-CKD studies of empagliflozin and dapagliflozin, which are evaluating renal outcomes as the primary endpoint, are due to report within the next two years.
The recently reported CREDENCE study was a double-blind, randomised controlled trial comparing canagliflozin with placebo in people with type 2 diabetes and albuminuric diabetic kidney disease (Perkovic et al, 2019). The primary outcome was a composite of end-stage renal disease, doubling of serum creatinine and death from renal or cardiovascular causes. The 4401 participants enrolled in the study had an eGFR in the range of 30–90 mL/min/1.73 m2 and were already receiving treatment with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs). A significant 30% RRR for the primary outcome was demonstrated.
Thus, there is now strong evidence that SGLT2 inhibitors may reduce the progression of diabetic nephropathy in type 2 diabetes, and that this benefit (like the cardiovascular benefits) persists at low eGFR.
The mechanism of renoprotection is thought to arise from vasoconstriction of the afferent glomerular arteriole and subsequent reduction in intraglomerular pressure, which protects the glomerular basement membrane, reducing albuminuria (Anders et al, 2016; Cherney et al, 2017). It is noteworthy that the renoprotection from ACE inhibitors and ARBs operates via vasodilation of the efferent arteriole of the glomerulus, and so the renoprotective effects of these agents act in a complementary manner to those of the SGLT2 inhibitors.
Side effects
The problem of diabetic ketoacidosis
As usage of the SGLT2 inhibitors became more widespread, it became apparent that they were associated with a small increase in risk of diabetic ketoacidosis (DKA), a potentially life-threatening complication (Rosenstock and Ferrannini, 2015; Peters et al, 2015; Fralick et al, 2017). A significant proportion of cases were associated with ketosis-prone diabetes (type 1 diabetes, latent autoimmune diabetes in adults, pancreatogenic diabetes, etc.). An important feature of DKA associated with SGLT2 inhibitors is that the complication may occur at only moderately elevated blood glucose levels (<14 mmol/L), in which case it is termed euglycaemic DKA. Regulatory bodies in Europe and the UK have issued warnings over the risk of DKA when using SGLT2 inhibitors (European Medicines Agency [EMA], 2016; MHRA, 2016). The EMA, however, concluded that the benefits of SGLT2 inhibitors continue to outweigh the risks in treatment of type 2 diabetes.
Situations predisposing to DKA in people taking SGLT2 inhibitors (typically where there is a relative insulin deficiency) are listed in Box 3 (EMA, 2016; MHRA, 2016; Wilding et al, 2018).
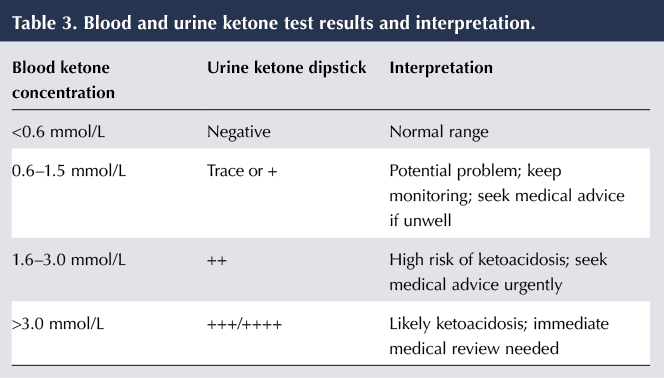
Symptoms of DKA include excessive thirst and urination, nausea and vomiting, abdominal pain, difficulty breathing, abnormal fatigue, confusion and a fruity smell of the breath. If individuals do develop these symptoms, they should seek immediate medical advice. Whilst blood glucose levels should also be checked, the key test is for blood ketones (preferable to urinary ketone testing). The implications of blood ketone levels are outlined in Table 3 but should always be interpreted in the light of the prevailing clinical picture.
If DKA is suspected then the SGLT2 inhibitor should be discontinued immediately. In the case of acute illness, dehydration or planned surgery, the SGLT2 inhibitor can be recommenced following recovery, but if there is no clear precipitating factor then it should not be restarted (EMA, 2016; MHRA, 2016).
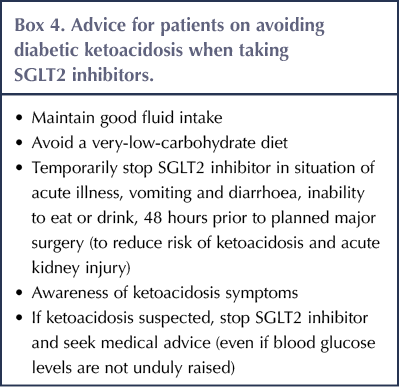
Individuals with type 2 diabetes taking SGLT2 inhibitors need to be advised how to avoid DKA (see Box 4), how to recognise it and what action to take if DKA is suspected. Clinicians need to be aware of the importance of testing for ketones if DKA is a possibility, even if blood glucose readings are not substantially elevated.
Genitourinary infection
The glycosuria induced by SGLT2 inhibitors predisposes to genital fungal infection and urinary tract infection (UTI). A meta-analysis of trials comparing SGLT2 inhibitors with placebo or other medications for type 2 diabetes showed that the class was associated with a five-fold increase in genital mycotic infections and a more modest 40% increase in UTIs (Vasilakou et al, 2013).
Candidal vulvovaginitis and balanitis are relatively common side-effects that are generally easily treated with topical (e.g. miconazole or clotrimazole cream for several days) or oral (e.g. fluconazole stat dose) antifungal agents. In most cases, they do not lead to treatment discontinuation. The strongest predictors of genital fungal infection are being female and having a previous history of infection (Thong et al, 2018). Recurrent and/or severe fungal infections may necessitate cessation of SGLT2 inhibitor therapy, although some individuals may choose to take prophylactic treatment (e.g. fortnightly fluconazole) in order to preserve the benefits achieved with SGLT2 inhibition.
The majority of UTIs are mild to moderate in severity (not upper UTIs) and respond well to standard therapy.
Fournier’s gangrene
Fournier’s gangrene is a necrotising fasciitis of the genitalia and perineum (predominantly in men) which is rare but potentially life-threatening. Diabetes is a known risk factor for Fournier’s gangrene, but post-marketing reports have raised the possibility of an association of this condition with use of SGLT2 inhibitors. The US Food and Drug Administration (2018) and the MHRA (2019) have issued warnings in this regard.
People taking SGLT2 inhibitors should be warned to seek urgent medical attention if they experience severe pain, tenderness, erythema or swelling in the genital/perineal area, along with fever or malaise. If Fournier’s gangrene is suspected then the SGLT2 inhibitor should be stopped immediately and urgent treatment commenced with antibiotics and, where necessary, surgical debridement (MHRA, 2019).
Diuretic side-effects
The glycosuria induced by SGLT2 inhibitors generates an osmotic diuresis that leads to more frequent voiding, which can be troublesome. There may be associated volume depletion side-effects, notably postural hypotension, which could be problematic in the elderly, who are more prone to dehydration. Thus, caution should be exercised when using SGLT2 inhibitors with diuretics and, generally, advice from the Summaries of Product Characteristics is to avoid combination with loop diuretics. Due to postural hypotension and the risk of acute kidney injury following circumstances of hypovolaemia/dehydration, the licences advise avoiding dapagliflozin in people aged >75 years and empagliflozin in those aged >85 years.
Amputation and fracture risk
In CANVAS, an increased risk of lower limb amputation was observed with canagliflozin (6.3 events per 1000 person-years) compared with placebo (3.4 events per 1000 person-years. Overall, 71% of those affected had the highest amputation at the level of the toe or metatarsal (Neal et al, 2017). This increased risk was associated with previous amputation and the presence of peripheral vascular disease.
Whether or not this represents a class effect remains uncertain. Although the CVOTs of dapagliflozin and empagliflozin did not show this increased risk of amputation, the EMA determined that a warning should be included in the Prescribing Information for all drugs in the class (EMA, 2017).
NICE (2015) and MHRA (2017) have provided advice on reducing the risk of lower limb amputation when using SGLT2 inhibitors in type 2 diabetes:
- Check feet regularly and follow preventative care.
- Report any foot infection or ulceration.
- Avoid initiation of SGLT2 inhibitors if active foot ulceration or if previous lower limb amputation.
- Be cautious about initiating SGLT2 inhibitors in people with peripheral vascular disease.
- Consider stopping SGLT2 inhibitors in cases of foot ulceration, osteomyelitis or gangrene.
Fracture
In the CANVAS study, which pooled data from two trials, CANVAS and CANVAS-R, a 26% increased risk of fracture was found with canagliflozin, with reduced bone mineral density at the hip, but not at other sites (Neal et al, 2017); however, this was only apparent in the CANVAS trial, not CANVAS-R. It has been postulated that the increased fracture risk may have been due to a greater occurrence of falls secondary to postural hypotension arising from the volume-depleting effects of canagliflozin. No change in incidence of fracture was evident in the CVOTs of dapagliflozin and empagliflozin (Zinman et al, 2015; Wiviott et al, 2019).
An observational study of over 17 000 individuals with type 2 diabetes taking SGLT2 inhibitors found that, compared with glucagon-like peptide-1 receptor agonist therapy, SGLT2 inhibitor use was associated with an increased risk of lower limb amputation but not bone fracture (Ueda et al, 2018).
Importantly, the CREDENCE trial, in contrast to CANVAS, did not find any increased risk of amputation or of fracture in those treated with canagliflozin versus placebo (Perkovic et al, 2019).
Thus, at this stage, the data on fracture risk are inconsistent, and it is hard to draw firm conclusions (Wilding et al, 2018).
Guidelines
The NICE (2015) NG28 guideline recommends SGLT2 inhibitors as a second-line option to metformin should HbA1c rise to 58 mmol/mol (7.5%), and places the class on an equal footing with dipeptidyl peptidase-4 inhibitors, sulfonylureas and pioglitazone; the appropriate option is to be selected on the basis of an individualised, patient-centred approach. Additionally, SGLT2 inhibitors could be considered as an option in triple therapy, including in combination with insulin. A NICE technology appraisal (TA390) also includes the possibility of using canagliflozin, dapagliflozin or empagliflozin as monotherapy for glycaemic control when diet and exercise are insufficient and metformin is not tolerated or is contraindicated (NICE, 2017). Thus far, ertugliflozin has been recommended as an option for monotherapy or dual therapy in combination with metformin for glycaemic control in type 2 diabetes (NICE, 2019), while advice on triple therapy is awaited.
The SIGN (2017) guidelines advocate a similar stepwise approach to treatment of hyperglycaemia in type 2 diabetes, but go a step further in recommending the preferential use of SGLT2 inhibitors as a dual therapy option in those with established CVD, based on the positive findings of the EMPA-REG OUTCOME and CANVAS trials.
More recently, the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) joint consensus report on management of hyperglycaemia in type 2 diabetes has further promoted the use of SGLT2 inhibitors based on assessment of individual circumstances according to comorbidities and needs (Davies et al, 2018; Down, 2018). Thus, following diet/lifestyle and use of metformin, the use of SGLT2 inhibitors for glycaemic control (provided eGFR is >60 mL/min/1.73 m2) is prioritised for those with:
- Established atherosclerotic cardiovascular disease (ASCVD)*
- Established ASCVD where heart failure coexists or is of special concern*.
- Chronic kidney disease (CKD) with or without ASCVD to reduce CKD progression.
- A compelling need to avoid hypoglycaemia.
- A compelling need to avoid weight gain. *The cardiovascular benefits were seen in the empagliflozin and canagliflozin CVOTs. Following the publication of the ADA/EASD consensus statement, the dapagliflozin CVOT showed that heart failure benefits extended to those without established ASCVD.
NICE has recently announced that it will be updating the NG28 guideline to take into account the data that have emerged since 2015 (Down, 2019).
SGLT inhibitors in type 1 diabetes
The mechanism of action of SGLT2 inhibitors suggest that they may be effective as glucose-lowering agents in type 1 diabetes, with weight loss as an added benefit to counter the weight gain expected with intensive insulin treatment. Evidence supporting this approach has been steadily accumulating but associated risks, notably that of DKA, will need to be carefully monitored (McCrimmon and Henry, 2018).
Two clinical trials, DEPICT-1 and DEPICT-2, demonstrated that adding dapagliflozin to insulin in people with type 1 diabetes led to improved glycaemic control (HbA1c reduction around 4 mmol/mol [0.4%]), weight loss (around 3% of body weight), reduction in total insulin dose and increased time within the target glycaemic range of 4–10 mmol/L, with no increased risk of hypoglycaemia (Dandona et al, 2018; Mathieu et al, 2018). However, the risk of DKA was increased (event rates up to 4.0% vs 1.9% with placebo in DEPICT-1) and, as expected, so was the risk of genital tract infection.
Sotagliflozin is a dual SGLT1/SGLT2 inhibitor that has been investigated in Phase 3 clinical trials as an adjunct to insulin in people with type 1 diabetes. The three inTandem trials demonstrated similar benefits to the DEPICT trials (Garg et al, 2017; Buse et al, 2018; Danne et al, 2018). A meta-analysis of randomised controlled trials comparing sotagliflozin with active comparators or placebo in adults with type 1 diabetes confirmed these benefits but reported that the risk of DKA was increased (relative risk, 3.93) and noted side-effects of genital tract infection, diarrhoea (due to effects of SGLT1 inhibition on the gut) and volume depletion (Musso et al, 2019). An important observation with inTandem3 was that individuals using insulin pumps were more likely to experience severe hypoglycaemia or DKA compared with those receiving subcutaneous injections (Garg et al, 2017).
In the EASE trials, empagliflozin showed improved glycaemic control and weight reduction in individuals with type 1 diabetes without increasing hypoglycaemia. DKA incidence was increased (except at a very low dose), as were genital infections (Rosenstock et al, 2018).
In light of the above evidence, both dapagliflozin and sotagliflozin have been approved by the EMA for use in adults with type 1 diabetes and a BMI >27 kg/m2 when insulin alone does not provide adequate glycaemic control.
When to use SGLT inhibitors in type 1 diabetes
The decision to use SGLT inhibitors in type 1 diabetes will need to be carefully judged. Glycaemic and weight benefits seem clear, but these are modest and will need to be balanced against the (low) risk of DKA – a potentially fatal complication. Initiation should only be under the supervision of a diabetes specialist.
A strong emphasis needs to be placed on patient education (see Box 4), particularly in relation to recognition of symptoms and signs of DKA (and recognising that this may occur at relatively low levels of glycaemia: <14 mmol/L); the use of ketone monitoring and interpretation (see Table 3); and what action to take in response to these observations. Patient selection will be crucial. SGLT inhibitors should be avoided in people who struggle to adhere to their insulin regimen and those who have had previous episodes of DKA (Llano et al, 2019).
Primary care professionals will need awareness of the risk of DKA and the need to discontinue SGLT inhibitors when DKA is suspected and during acute illness (sick day rules), when the risk is of DKA is higher; in these situations, fluid management, maintenance of carbohydrate intake and increased insulin dosage are important (insulin should never be stopped in these circumstances).
NICE guidance on the use of dapagliflozin, sotagliflozin and empagliflozin as adjunctive treatment to insulin in type 1 diabetes is in development.
Conclusion
Recent evidence has propelled the SGLT2 inhibitors to a position of increasing importance in the management of type 2 diabetes. In a variety of situations (established cardiovascular disease, heart failure, diabetic nephropathy), they may be considered a preferred option as an add-on to metformin therapy to improve glycaemic control, with associated benefits including weight loss, low risk of hypoglycaemia and versatility to use with other agents. Genital fungal infection is a common side effect, and DKA a rarer but serious complication of treatment. Use in primary care looks set to increase.
The utility of SGLT inhibitors in type 1 diabetes has been demonstrated, although use in practice will require cautious patient selection by a diabetes specialist and, subsequently, careful monitoring because of the increased risk of DKA.









Helping homeless adults to overcome the challenges of managing their condition.
16 Apr 2024