Hot topics in primary care
Cardiorenal and heart failure benefits of SGLT2 inhibitors
Kevin Fernando (North Berwick Health Centre) used a case-based approach to lead primary care teams through the landmark trials supporting sodium–glucose cotransporter 2 inhibitor (SGLT2i) use and how we can translate the trials into our day-to-day practice. He encouraged clinicians not just to focus on glycaemic lowering but also to consider significant comorbidities such as atherosclerotic cardiovascular disease (ASCVD), heart failure (HF) and diabetic kidney disease (DKD).
He reminded us that the NICE guideline on type 2 diabetes management is currently out of date, with new guidelines being developed, and highlighted the key recommendations in the pharmacological update from SIGN (2017) recommending that in those with established CVD, we should consider the use of an SGLT2i or glucagon-like peptide-1 receptor agonist (GLP-1 RA) with proven cardiovascular morbidity and mortality benefit.
Dr Fernando encouraged his audience to consider SGLT2is as first-line therapy after metformin in many people with type 2 diabetes due to their proven benefits beyond glucose lowering. He outlined the key findings from the landmark studies demonstrating reductions in major adverse cardiovascular events (MACE; cardiovascular death, non-fatal myocardial infarction or stroke) in the EMPA-REG OUTCOME and CANVAS programmes, and the reduction in the co-primary endpoint of hospitalisation for heart failure (HHF) and cardiovascular death in the DECLARE-TIMI 58 trial, as well as the significant reduction in HHF in all three trials.
The CREDENCE trial in those with type 2 diabetes at high risk of cardiovascular and renal events demonstrated a significant reduction in the combined renal outcome, resulting in a licence change in July 2020 for canagliflozin to treat DKD in those living with type 2 diabetes. DAPA-CKD, a renal outcome study similar to CREDENCE with dapagliflozin, reduced major adverse renal events in those with and without type 2 diabetes, and a licence change is currently awaited.
The DAPA-HF study demonstrated significant reductions in a combination of worsening HF and cardiovascular death in those with and without type 2 diabetes, with reductions in both cardiovascular and all-cause mortality. This resulted in a licence change for dapagliflozin in November 2020 allowing use in those with HF and reduced ejection fraction (HFrEF; ejection fraction ≤40%) in adults with or without type 2 diabetes. Finally, the EMPEROR-Reduced trial in those with HFrEF with and without type 2 diabetes demonstrated reductions in cardiovascular death and HHF in those treated with empagliflozin. A UK licence change is awaited.
How to translate this into our practice
Dr Fernando recommended we follow the 2019 update of the American Diabetes Association/European Association for the Study of Diabetes Consensus Report on type 2 diabetes glucose management, which is already widely used across the UK (Buse et al, 2020). For those with ASCVD, whether or not glycaemic targets are achieved, a GLP-1 RA with proven cardiovascular benefit or an SGLT2i with proven cardiovascular benefit (if the eGFR is adequate) are recommended as the first-line add-on to metformin. However, if HF or CKD predominates then, irrespective of whether glycaemic targets are achieved, an SGLT2i with proven benefit for HF or CKD, respectively, should be added. The GPnotebook Shortcut for prescribing glucose-lowering therapies in those with renal problems has been updated to include the current licence changes for dapagliflozin and canagliflozin.
Finally, Dr Fernando reminded his audience that, although he had focused on the benefits of drug therapy, we should also always discuss the known adverse events associated with these newer classes of drugs with people before prescribing. We should also remember that lifestyle measures have much to offer in reducing the risk of cardiorenal events.
GPnotebook Shortcuts
- What next after metformin?
- Prescribing for people living with type 2 diabetes and renal impairment
- Diagnosis and classification of diabetes for primary care
Ethnicity, diabetes and COVID-19
Kamlesh Khunti (University of Leicester) shared an overview of the impact which COVID-19 has had on people with diabetes – particularly those from black, Asian and minority ethnic (BAME) groups – and the delivery of diabetes care during the last year. He reminded the audience that public health crises pose both direct and indirect risk to people with diabetes, picking out four key areas to discuss: outcomes, access to care and management, adherence to treatment, and the psychosocial impact. For example, people with diabetes are known to experience worse outcomes during a public health crisis, as has occurred during COVID-19. He stressed the disruption both to contact with healthcare professionals and to the community support systems and self-management options that are so important to people with diabetes, and the likely detrimental effects which this will have on management of both their diabetes and the associated comorbidities in the short and longer term. For example, remote rather than face-to-face healthcare consultations, lack of retinal screening and management of diabetic eye disease, stress from mental health conditions and diabetes distress may all impact on people with diabetes. Drug treatments and self-management (e.g. physical activity) have been much harder during the pandemic.
Professor Khunti shared some of the global experiences and resources developed during the pandemic. A World Health Organization global survey to identify chronic disease and comorbidities most impacted by COVID-19, due to the reduction in accessible care, demonstrated that diabetes was significantly more impacted than the other chronic diseases, chosen by nearly 40% of respondents in the survey. When respondents were asked for the two most common co-occurring chronic diseases impacted by reduction in care during the pandemic, diabetes and hypertension was the commonest response, followed by diabetes and COPD, with diabetes and HF and diabetes and CVD also in the top 5 combinations (Chudasama et al, 2020).
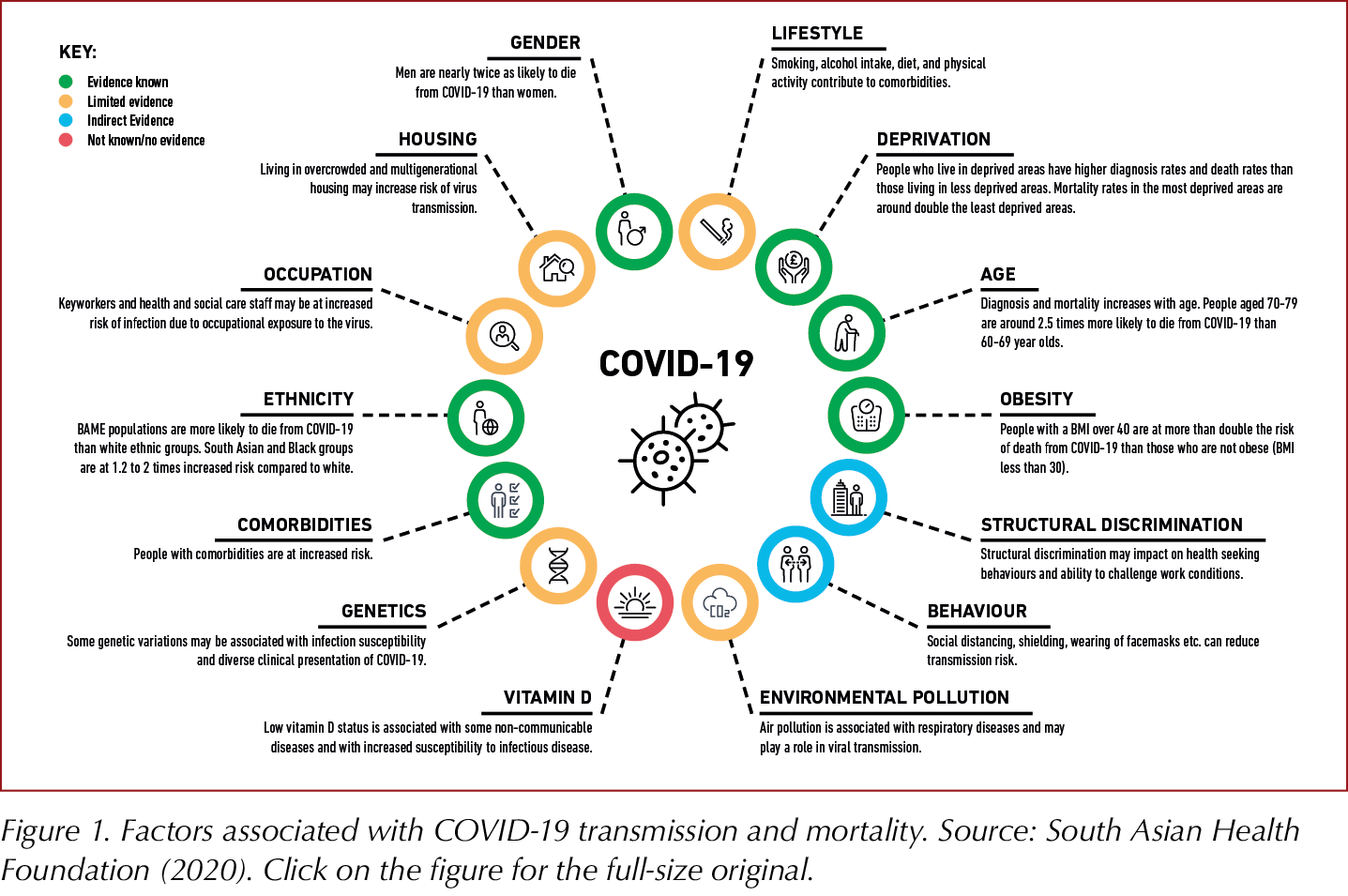
Sharing the South Asian Health Foundation (SAHF) report on COVID-19, Professor Khunti outlined disparities in and risk predictors for COVID-19 outcomes, as summarised in Figure 1. He concluded with five key messages that we should all know in relation to COVID-19 and people with diabetes:
- It is unclear if people with diabetes are more likely to contract COVID-19.
- Current data suggest that COVID-19 is associated with worse outcomes in people with diabetes.
- There is an association between blood glucose control and COVID-19 outcomes, with worsening control associated with worsening outcomes.
- The COVID-19 pandemic is likely to have long term impact on people with diabetes due to disruptions, including stress and changes to routine care, diet and physical activity.
- Encourage COVID-19 vaccination for all people with diabetes and their carers, particularly in BAME groups in which uptake may be lower and risk from COVID-19 higher.
Resources
- COVID-19 in BAME populations: SAHF recommendations
- SAHF resources on COVID-19 and vaccination in South Asian languages
Diabetes and psychiatric disorders
Abbi Lulsegedd (Health 121 Ltd) began by reminding the audience that people with diabetes are three times more likely to have depression than those without diabetes and that diabetes is also associated with bipolar and mood disorders, anxiety states and eating disorders, with 10% of those with type 2 diabetes suffering from an eating disorder.
The interaction of diabetes and depression is bidirectional, with depression occurring in around 20% of people with diabetes and depression associated with a 60% increased risk of type 2 diabetes. Depression is associated with a higher number of cardiovascular risk factors and increased risk of diabetes complications.
Dr Lulsegged shared data from the US-based, longitudinal cohort study, the Multi-Ethnic Study of Atherosclerosis, examining the rate of incident diabetes related to depression and of incident depression in those with diabetes (Golden et al, 2008). The incidence of type 2 diabetes was significantly higher in those with elevated depressive scores (22.0 versus 16.6 per 1000 person-years), independent of sociodemographic, economic and metabolic factors. Amongst those on treatment for type 2 diabetes, the incidence of depression was 61.9 per 1000 person-years, significantly higher than the 36.8 per 1000 person-years seen in those with normal glucose tolerance, while there was a lower incidence of depression in those with impaired glucose regulation or untreated type 2 diabetes.
Some of the risk factors associated with depression that can increase the risk of type 2 diabetes include comfort eating, including sugary and fatty foods, eating calorie-dense foods, reduced physical activity, smoking, the stress response, inflammation, and reduced concordance with lifestyle changes and medications.
Diabetes distress
Diabetes distress can be defined as “emotional distress resulting from living with diabetes and the burden of relentless daily self-management”. This can involve worry about the future and the possibility of development or worsening of complications, and experiencing feelings of guilt and anxiety when diabetes management goes off track. It affects around 25% of those with type 1 diabetes and 20% of those with type 2 diabetes. Although 65% of people with diabetes have no diabetes distress or depression, 12% have depressive symptoms only, 13% have diabetes distress and depressive symptoms, and 10% have diabetes distress alone. The course fluctuates over time and peaks at diagnosis and when complications develop or worsen. Clues include not attending appointments, poor control of diabetes and ineffective coping mechanisms (e.g. comfort eating). Questions which can be useful in identifying diabetes distress include:
- What is the most difficult part of living with diabetes for you?
- What are your greatest concerns about your diabetes?
- How is your diabetes getting in the way of other things in your life right now?
The stress response is generally protective in acute situations, but sustained or frequent stress can lead to exposure to chronically elevated cortisol levels, which in turn can cause depression, anxiety, sleep disorders, carbohydrate cravings, weight gain, hypertension, poor immune function, irritable bowel syndrome and reduced memory.
The relationship between cortisol and depression was explored further. Disturbances of the hypothalamic–pituitary–adrenal axis are most pronounced in those with treatment-resistant depression. The elevated cortisol levels are associated with pro-inflammatory cytokine responses, and these in turn are associated with visceral adiposity, insulin resistance and diabetes. Treatment of the depression seems to be associated with reductions in high-sensitivity C-reactive protein levels.
Depression, diabetes and sleep
Insomnia is common in those with depression and is associated with diabetes risk through various pathways that include a role for melatonin. Melatonin helps regulate glucose-stimulated insulin secretion, improves sleep quality, and reduces oxidative stress and inflammation. It is not clear whether improved sleep could reduce depression risk in people with diabetes. The Nurses’ Health Study demonstrated an association between lower blood and urine levels of melatonin at baseline and increased risk of type 2 diabetes: 9.27 cases per 1000 person-years in the group with the lowest levels of melatonin versus 4.27 per 1000 person-years in the highest melatonin group (McMullan et al, 2013).
In a small Israeli study, treatment with prolonged-release melatonin 2 mg in people with type 2 diabetes and insomnia demonstrated improvements in sleep and, after 5 months, significantly improved HbA1c from 76 to 69 mmol/mol (9.1% to 8.5%).
Managing depression in diabetes
Many studies have explored the relationship between antidepressant use and diabetes, particularly in relation to weight gain. Risk of incident type 2 diabetes varies from a hazard ratio of 1.25 with selective serotonin reuptake inhibitors (SSRIs) to 1.82 with mixed antidepressants. Risks of increasing weight by ≥5% are greater in those taking antidepressants, and SSRIs and mirtazapine increase risk, especially in the first 4 weeks in the case of mirtazapine. There is evidence from one study that sertraline may help improve glycaemic control, but additional exploration is needed. Use of repetitive transcranial magnetic stimulation instead of antidepressants has been evaluated by NICE and is considered safe, although headaches, pain and facial twitching may occur and NHS use is limited.
We should ask people to be honest with us rather than pursuing a stoical approach. Even in a short consultation we can gather information about how depressed they are. Dr Lulsegged then shared and discussed the stepped care model to support management decisions (Figure 2).
Around 60 000 new type 2 diabetes cases missed in 2020
Matthew Carr (University of Manchester) examined the indirect effects of the COVID-19 pandemic on the diagnosis and monitoring of type 2 diabetes in 2020, using anonymised primary care data from the Clinical Practice Research Datalink (CPRD). Compared with historical data from the previous 10 years, there was a 70% drop in the rate of new type 2 diabetes diagnoses in April 2020 and, while the number of diagnoses gradually rose to average levels by the end of the year, the levels were greatly reduced throughout the majority of the year, and this shortfall has yet to be addressed.
Extrapolating these data to the whole of the UK, an estimated 60 000 new type 2 diabetes diagnoses have been missed, and this may even have been an underestimate if changes in diet and physical activity during the various lockdowns have led to an above-average incidence of the condition.
The drop in diagnoses was largest in England compared with the other UK nations; however, the same pattern was observed across the UK. New metformin prescriptions also followed the same pattern, suggesting that the issue is genuinely one of missed diagnoses rather than a lack of coding.
Among people with pre-existing type 2 diabetes (approximately 600 000 captured within the CPRD), similar patterns emerged when analysing rates of the key care processes and treatment escalation (new prescriptions of glucose-lowering and antihypertensive drugs). In contrast, repeat prescription rates did not fall.
Given the consequences of delayed diagnosis on diabetes-related complications, there is an onus to resume health checks and diabetes screening as quickly as possible. A new Quality and Outcomes Framework indicator, The percentage of patients with non-diabetic hyperglycaemia who have had an HbA1c or fasting blood glucose performed in the preceding 12 months, should help motivate practices to undertake this task.
The findings have since been published in The Lancet Diabetes & Endocrinology (click here to access).
Weight management issues
Rachel Batterham (University College London) outlined the barriers that people with type 2 diabetes and excess weight face in accessing treatment. These include low prioritisation and funding for weight management services, a widespread lack of understanding of the physiology of obesity, and the stigma that results. She highlighted a number of ways healthcare professionals can help.
Signposting to patient support groups is a highly effective way to help overcome barriers to treatment. Such groups include Obesity UK, the European Coalition for People Living with Obesity, the Obesity Action Coalition and the Obesity Empowerment Network UK.
Professor Batterham also recommended that healthcare professionals use the 5As strategy (Ask, Assess, Advise, Agree, Assist) to discuss weight with their patients (Table 1).
Dietary fibre
The Harry Keen Rank Nutrition Lecture was delivered by Denise Robertson (University of Surrey) and was titled “The 21st century ‘rediscovery’ of dietary fibre: From bedside to bench to bacteria, and back again”. Dr Robertson explored the importance of fibre for health and the mechanisms underlying these health benefits, particularly in relation to metabolic effects, and explored how we can translate these findings into benefits for real people.
Historically, humans ate 100 g of fibre daily – much more than the 30 g recommended daily intake today, let alone the 15–20 g average consumption – and this means we have the capacity to increase fibre intake, and most would benefit from doing so.
Type 2 diabetes became more common from 1956, when white flour was reintroduced to replace the very high-fibre “National Loaf” used during the war years. In 1971, Burkitt and Trowell identified that a single nutrient may influence a variety of chronic diseases, such as bowel cancer and heart disease, and they proposed that fibre was that nutrient. Until 2015, fibre recommendations were made only in relation to gut transit time and preventing constipation, and cardiometabolic benefits of fibre were not discussed.
A recent systematic review and meta-analysis looking at high-fibre diets and diabetes (type 1, type 2, gestational and pre-diabetes) demonstrated improvements in all the diabetes parameters studied with high- versus low-fibre diets (Reynolds et al, 2020). The relative risk of all-cause mortality over 8.8 years of follow-up was 0.5 (95% confidence interval, 0.35–0.86) between the highest (30–35 g) and the lowest (14 g) daily fibre intakes.
Fibre fermentation in the gut is important – it is broken down by colonic bacteria to make short-chain fatty acids (SCFAs; butyrate, propionate, acetate) which feed colonocytes, decrease local inflammation and can be absorbed to interact with peripheral tissues to limit fatty acid release and, ultimately, improve insulin sensitivity in muscle and liver.
The microbiome has been linked to obesity, intestinal and systemic inflammation, glucose control, pancreatic function and appetite regulation. Discovery of the human microbiome has changed how scientists see fibre and it is now possible to link it to most diseases. Fibre is recognised as the most consistent way to change the microbiome, and the preferred fuel of the microbiome is carbohydrate and fibre. In the absence of enough fibre in the diet to produce beneficial SCFAs in the colon, bacteria will ferment protein, producing toxic by-products such as secondary bile acids and phenols, which increase gut wall permeability, allowing increased absorption of lipopolysaccharide and other molecules (Yang and Yu, 2018). We need both protein and fibre in the diet, and getting the balance right is important. Plant protein has advantages over animal protein since the protein is pre-packaged with fibre.
Dr Robertson also sought to answer key questions about dietary fibre. Can we “out-fibre” a bad diet? A high-fibre diet may be able to protect gut health but it is unlikely that fibre can overcome the other effects of a poor high-fat or overprocessed diet. How can we increase fibre intake? Could we just administer SCFAs orally? Unfortunately, these taste and smell horrible and are absorbed too high up in the gut. Binding SCFA to inulin to make it an ester improves palatability and may help carry it into the colon, and studies are ongoing; however, it is possible that there is no additional benefit over inulin alone.
Resistant starch is structurally a starch but functionally a fibre. It is found in whole grains, beans and bananas, is produced during food processing, and is achieved by cooking and then chilling starchy foods such as potatoes and pasta. When added to other foods, resistant starch may influence the postprandial glucose peaks; this is being explored in research studies using a starch swap rather than a food swap. The goal would then be to produce high-fibre foods which were cost-effective and acceptable tasting.
Other topics of interest include how to fill the fibre gap and encourage people to eat more fibre, and whether we can change the gut bacteria with probiotics or with faecal transplant, and whether that will provide metabolic benefits in diabetes.
Dr Robertson concluded that the health benefits of fibre are clear – it is good and we all need to eat more of it. Although current dietary recommendations are 30 g/day, she proposed there is no reason not to aim higher, and this may be even more beneficial.
What should we recommend
- Increase of 15 g fibre per day initially, aiming for 35 g/day total.
- Build up fibre gradually to avoid side effects.
- If irritable bowel syndrome, limit short-chain fibre such as FODMAP foods.
- Eat a variety of fibre types to gain all health benefits.
- Avoid high animal protein intake and balance with fibre to allow fibre fermentation in the colon, thus avoiding protein fermentation, which produces toxic metabolites and increases gut permeability.
- Resistant starch (e.g. potatoes and pasta that have been cooked and cooled) may allow addition of fibre surreptitiously.
Counterbalance study: Cardiovascular effects of weight loss in type 2 diabetes
New results from the DUK-funded Counterbalance study reveal that putting type 2 diabetes into remission through weight loss can have the additional benefit of reducing the risk of cardiovascular diseases such as a heart attack or stroke (Poster P25).
Thirty participants underwent an 8-week very-low-calorie diet followed by a stepwise return to an isocaloric diet and a 6-month weight maintenance phase. At 6 months, mean weight fell from 98 kg to 85 kg, and 13 participants achieved remission (HbA1c <48 mmol/mol and fasting plasma glucose <7.0 mmol/L after withdrawal of glucose-lowering drugs at the start of the trial).
Ten-year cardiovascular risk (QRISK3 model) decreased significantly after weight loss both in those who achieved remission and in those who did not, but non‐responders remained at higher risk (15.0% vs 5.8%). At 6 months, body weight and subcutaneous and visceral fat volumes decreased in both groups, and plasma biomarkers of cardiovascular risk improved after weight loss, irrespective of remission status.
The authors concluded that weight loss markedly decreases cardiometabolic risk, particularly when remission of diabetes is achieved. Normalisation of 10-year cardiovascular risk and heart age is possible after substantial dietary weight loss and particularly if remission of diabetes is achieved.
Metformin in pregnancy: Pros and cons
Fidelma Dunne (National University of Ireland Galway) outlined the evidence for and against using metformin in pregnancy, in women with either pre-existing type 2 diabetes or gestational diabetes. Although its use in pregnancy is an off-label indication, metformin has a wealth of safety data from its use over the last 60 years, it is simple to use and generally well tolerated, and it has a low cost.
Large international registry studies have found no evidence of any increased risk of congenital abnormalities with first-trimester exposure to metformin therapy. Randomised controlled trials have shown short-term pregnancy benefits compared with placebo, including lower rates of excessive maternal weight gain and improvements in glycaemic control, as well as lower rates of macrosomia and neonatal hypoglycaemia.
In a direct comparison with insulin in women with gestational diabetes, the metformin group had better maternal postprandial glucose and less maternal weight gain, with less neonatal hypoglycaemia (Rowan et al, 2008). Maternal preference was also greater for metformin, and medication concordance was higher.
However, while metformin reduces the likelihood of large-for-gestational-age offspring, it also increases the rate of small-for-gestational age children. Longer-term follow-up at 2–10 years of age also showed that children exposed to metformin in the womb had higher mean BMIs and other measures of adiposity (Hanem et al, 2019). Compared with insulin, there was a higher risk of preterm birth with metformin.
When asked for her opinion, Professor Dunne suggested she would avoid metformin in cases of fetal abnormalities, small fetal size (<10th centile), twins, or renal or liver function abnormalities in the mother. She would also suspend treatment if fetal growth slowed and fell below the 10th centile. She also suggested taking a detailed history of the mother and avoiding metformin if there was any suspicion of type 1 or monogenic diabetes.






Jane Diggle discusses emotional health and diabetes distress, and offers some tips for discussing this in our consultations.
11 Nov 2025