Updated ADA/EASD type 2 diabetes consensus published
The EASD and the American Diabetes Association (ADA) launched their updated Consensus Report on the Management of Hyperglycaemia in Type 2 Diabetes at the conference. The new report has an increased focus on managing weight loss, on person-centred care and on equity of care, as a result of feedback provided in response to the draft shared with delegates at the ADA conference in June 2022. It updates the previous consensus report (2018) and its update (2019), and this new document was published in both the EASD’s journal Diabetologia and the ADA’s journal Diabetes Care to coincide with the conclusion of the presentation at the EASD meeting in Stockholm.
The update explores in detail how social determinants of health (SDOH) impact on effective management of hyperglycaemia and how equity of care can be improved. The person-centred focus is strengthened with guidance on language use; shared decision-making, with people with diabetes involved in all aspects of care decisions; and encouragement of people to be involved and take greater responsibility for their own care (Figure 1). Clinicians are reminded to take into account personal circumstances when planning and delivering individualised care. Patients and healthcare professionals are encouraged to adopt more aggressive care, including combination therapy from the time of diagnosis, and to ensure regular reviews and avoid therapeutic inertia.
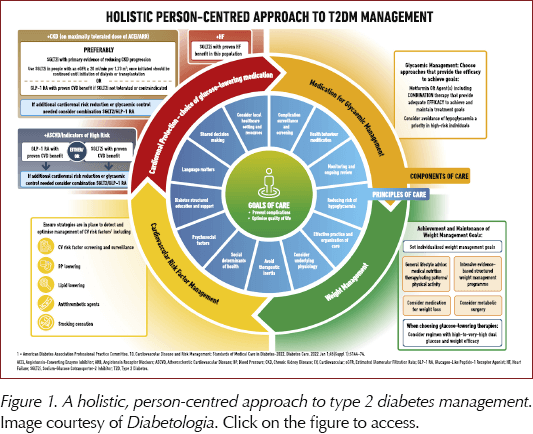
There is an increased focus on the importance of weight loss in managing hyperglycaemia, including review of the impact of medications, both glucose-lowering and those specifically studied to assist with weight loss (Figure 2). There is a holistic approach to lifestyle, involving guidance on nutrition, physical activity and, for the first time, a recommendation to achieve regular patterns of 6–9 hours’ sleep each night. The physical activity guidance is detailed, and includes not just the usual recommendations to achieve 150 minutes of moderate-to-vigorous physical activity per week combined with two sessions of resistance exercise, but also to break up sitting/sedentary time at 30-minute intervals with light exercise or resistance training, and the benefits of undertaking 500 extra daily steps.
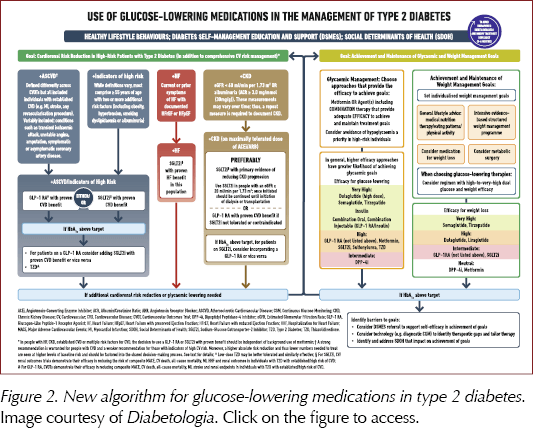
The Consensus is updated with evidence published since 2018, including studies of SGLT2 inhibitors, GLP-1 receptor agonists (including higher doses and oral GLP-1 RAs) and the new GIP/GLP-1 RA class, as well as the combinations of GLP-1 RAs with insulin. The benefits of these drugs on atherosclerotic cardiovascular disease, heart failure and chronic kidney disease, where applicable, are summarised, and guidance on their use has been updated to add clarity.
The update has been prepared by an international committee of experts that include Professor John Buse (University of North Carolina School of Medicine) and Professor Melanie Davies (Leicester Diabetes Centre) and their colleagues.
Click here to read the updated Consensus Report in full.
Further information
Click here to read our in-depth analysis of the changes to the guidance.
Click here for an at-a-glance factsheet on the updated lifestyle advice.
Diabetes and life expectancy – effects of demographics and lifestyle in Salford, UK
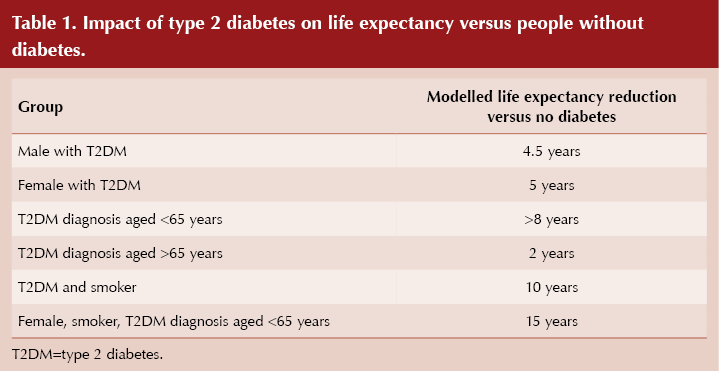
Surprisingly, type 2 diabetes appears to impact life expectancy in women more than in men, with reductions of 5 years and 4.5 years, respectively, compared to their counterparts without diabetes. Data from Salford, UK, presented at the Annual Meeting, showed greater impacts of type 2 diabetes on mortality in women than in men, in those aged <65 years at diagnosis compared with >65 years, and in smokers. Women with type 2 diabetes living in this community had an average of 4.84 excess life-years lost compared to women without diabetes.
During the 10 years studied, which preceded the COVID-19 pandemic, 3921 deaths occurred in people with diabetes compared to the 2135 expected, giving a standardised mortality ratio of 1.84, meaning the risk of early death was, overall, 84% higher in those with diabetes compared to the general population.
Salford is known to be one of the most disadvantaged areas of England, but even after adjustment for deprivation, women with type 2 diabetes had a 60% increased risk of early death and lived 5 years less than an average woman without diabetes, while men with the condition had a 44% increased risk of dying early and lived on average 4.5 years less than men without diabetes (Table 1). Smoking reduced life expectancy in those with diabetes by 10 years, and increased the risk of premature death by 2.5 times, compared with the general population.
Presenting the data, co-author Adrian Heald (Salford Royal Hospital) highlighted that their modelling suggests that type 2 diabetes has a greater effect on the life expectancy of women, smokers and those diagnosed under 65 years of age. He hopes that sharing these risks and their magnitude with people with type 2 diabetes may encourage them to stop smoking and motivate them to make other changes which can improve quality and quantity of life. He postulated that the surprising finding of the higher excess life-years lost in women compared to men could reflect changes in the LDL receptor following the menopause, lower prescription of statins in women or, possibly, decreased concordance with medications, but he stressed this finding needs further exploration.
The Salford Integrated Record study has been collecting and collating data from a population of around 270 000 people, with access to anonymised primary care records, outpatient attendances, admissions and deaths in this deprived area of the UK over the last 20 years. Standardised mortality rates and excess life-years lost were calculated over the years 2010–2020, ending prior to the pandemic. Data were adjusted for deprivation. Data interpretation is ongoing, assessing the impact of other parameters.
ReTUNE – type 2 diabetes remission in people of normal weight
The ReTUNE (Reversal of Type 2 Diabetes upon Normalisation of Energy Intake in the Non-obese) study, presented at the Annual Meeting, demonstrated that in people who develop type 2 diabetes despite being of normal weight (mean BMI, 24.8 kg/m2), weight loss of around 10% can result in diabetes remission in the same way it can help achieve this in people with overweight or obesity. Scans undertaken during the ReTUNE study demonstrated that those with type 2 diabetes had more visceral fat than healthy BMI-matched controls without the condition, confirming the same pathophysiology as in overweight and obese people who develop type 2 diabetes.
Twenty men and women, average age 59 years, participated in the Diabetes UK-funded study. The weight loss programme involved 2 weeks of an 800 kcal/day diet (soups, shakes and non-starchy vegetables), followed by 4–6 weeks of maintaining the weight loss. Participants completed one or two additional diet/maintenance cycles until they achieved weight loss of 10–15% and then maintained this weight loss to 12 months.
Participants lost an average of 7.7 kg (10.7% of their original weight) and average BMI reduced from 24.8 to 22.4 kg/m2 during the study. Over the course of 12 months, following achievement of weight loss, glucose levels reduced and insulin secretion gradually improved but did not return entirely to normal. High levels of satisfaction with the diet were recorded amongst participants.
Fourteen of the 20 study participants (70%) achieved remission, defined as HbA1c <48 mmol/mol for 6 months, off all glucose-lowering medication. Median time to remission was 8 weeks and weight was maintained from the final study visit until 12 months.
Special MRI scans were used to measure liver and pancreas fat in the study participants and healthy controls without type 2 diabetes, matched for BMI, age and sex. Scans demonstrated relatively normal liver fat levels of around 4.1% in those with type 2 diabetes at the start of the study, but this was around three times higher than liver fat in the healthy controls of similar BMI. Liver fat levels fell to 1.4%, close to the normal level for their BMI, after weight loss. Pancreas fat levels reduced from an average of 5.8% to 4.3% in those receiving the weight-loss diet, and beta-cell function returned towards normal. Visceral fat levels remained slightly elevated compared to controls without diabetes.
Weight loss-induced remission of type 2 diabetes was demonstrated previously in the DiRECT study in people with BMI over 27 kg/m2 who lost 10–15% of body weight (Lean et al, 2019). In that study, remission was also demonstrated to be associated with reductions in fat levels in the liver and pancreas.
The results of both studies support the “Personal Fat Threshold” theory proposed by Professor Roy Taylor and his team from Newcastle University. This posits that people who develop type 2 diabetes, at any weight, have exceeded their individual ability to safely store fat, resulting in increased storage in the liver and pancreas, causing beta-cell dysfunction and diabetes. Removing the fat from these organs by weight loss can support type 2 diabetes remission.
Around 15% of people who develop type 2 diabetes have a normal BMI, and this study demonstrates that the pathophysiology is the same as in those who are overweight or obese, and that weight loss is therefore key to removing excess fat from the liver and pancreas, and achieving remission. However, the study authors emphasised the importance of excluding other types of diabetes in this leaner group.
Professor Taylor and his team hope that raising awareness that type 2 diabetes is due to storing a little too much fat in the liver and pancreas, rather than being due to obesity per se, will reduce the stigma attached to developing the condition and encourage a paradigm shift amongst healthcare professionals.
Novel triple-acting agent shows promising effects on glycaemia and weight
Many of the newer glucose-lowering therapies have centred around the incretin hormones glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide (GIP), with DPP-4 inhibitors, GLP-1 RAs and the dual GIP/GLP-1 receptor agonist tirzepatide all in production.
Development is ongoing, and results of a Phase 1 study of LY3437943, a novel agent targeting the GIP, GLP-1 and glucagon receptors, were presented at the Annual Meeting. Seventy-two people with type 2 diabetes were randomised to varying doses of the study drug, or to dulaglutide or placebo, for up to 12 weeks.
By week 12, mean HbA1c decreased in all groups, with higher doses of LY3437943 showing significant placebo-adjusted reductions of up to 17.1 mmol/mol. Dose-dependent reductions in body weight, up to 8.96 kg greater than with placebo, were observed with the new agent. Although the authors cautioned against using cross-study comparisons, it appears that the reductions in HbA1c and body weight were greater even than those achieved with tirzepatide in its Phase 3 trials. Significant reductions in blood pressure were also seen in the LY3437943 groups.
As with the GLP-1 RA class, the most common treatment-emergent adverse events were gastrointestinal in nature and mostly mild in severity. However, some concern was expressed over increases in heart rate, with increases of 10 beats/min from baseline seen with the highest dose, roughly double that seen with tirzepatide and semaglutide in the SURPASS-2 study.
Brief summary: UKPDS at 44 years – do the metabolic and metformin legacy effects persist?
UKPDS (UK Prospective Diabetes Study) timeline:
• 1977–1997: 10 year randomised controlled trial (UKPDS Group, 1998):
- 2729 intensive SU/insulin; 1138 conventional with diet, 342 intensive overweight with metformin. Mean age 62 years at completion.
• 1997–2007: Observational post-trial monitoring study. Mean age 72 years at end (Holman et al, 2008).
• 2007–2021: NHS administrative follow-up. 10–12% of participants still in the study.
Comparison of SU/insulin intensive vs conventional control:
• 10 mmol/mol (0.9%) difference throughout 10 years of trial.
• Any diabetes-related endpoint: Consistent significant benefits; 10% relative risk reduction (RRR).
• Myocardial infarction: Not significantly reduced during the trial period; emerging significance by end of the monitoring period; 15% RRR.
• Microvascular disease: Highly significantly reduced throughout; 26% RRR at 44 years.
• All-cause mortality: Significant after post-trial monitoring; 11% RRR at 44 years.
• Cumulative hazard ratios confirm the legacy effect is stable over time.
Comparison of metformin intensive vs conventional control in overweight people:
• 6 mmol/mol (0.6%) lower HbA<sub>1c</sub> throughout trial. All endpoints significantly reduced except microvascular disease:
- Any diabetes-related endpoint: 19% RRR.
- Myocardial infarction: 31% RRR.
- Microvascular disease: Not significant at any stage.
- All-cause mortality: 25% RRR at 44 years.
Simulation model:
• A 50-year-old individual, newly diagnosed, with HbA1c 64 mmol/mol (8.0%). Compared with remaining at 64 mmol/mol for 20 years:
- If remains at 64 mmol/mol for 10 years then reduced to 53 mmol/mol (7.0%): 6.6% RRR in mortality.
- If 53 mmol/mol achieved early and maintained: 18.6% RRR in mortality – nearly three times higher.
Legacy effect = impacts that previous conditions have on current processes or properties
• Likely driven by lifetime exposure to hyperglycaemia, mediated possibly by oxidative stress, generation of advanced glycation end-products, epigenetic mechanisms leading to enhanced expression of pro-inflammatory genes (Lind et al, 2021).
• Larger metformin legacy effect despite smaller differences in HbA1c may reflect drug-specific protective effects, such as inhibition of inflammatory pathways.
Brief summary: Lifestyle, type 2 diabetes and dementia risk.
• T2DM is associated with increased risk of dementia.
• UK Biobank study conducted to determine whether healthy lifestyle factors could modify this risk.
• Lifestyle behaviour scores categorised as most, moderate and least healthy, based on these behaviours:
- Television viewing time.
- Sleep duration.
- Physical activity.
- Alcohol consumption.
- Processed and red meat intake.
- Fruit and vegetable intake.
- Oily fish intake.
- Smoking status.
• Prospective study, 369 627 participants, mean age 55.6 years, 54.6% women. Median follow-up 9.1 years after excluding the first two years.
• Risk of all-cause dementia:
- T2DM vs no diabetes: 28% higher.
- Least healthy vs most healthy lifestyle: 91% higher.
• Risk of all-cause dementia compared with controls (no T2DM, healthiest lifestyle):
- No T2DM but least healthy lifestyle: 36% higher.
- T2DM and least healthy lifestyle: 142% higher.
• Those with type 2 diabetes and the healthiest lifestyle had similar risk of dementia to the least healthy without type 2 diabetes.
• Conclusion: The associations between T2DM and dementia risk are accentuated by unhealthy lifestyle behaviours and partially attenuated by healthy behaviours.





Introducing the PCDO Society’s new podcast. In episode 1, we cover the updated NICE NG28 guideline on type 2 diabetes management.
24 Feb 2026