Diabetes is the commonest cause of chronic kidney disease (CKD) and end-stage kidney disease (ESKD; National Kidney Foundation, 2012; Renal Association, 2017). Diabetic kidney disease (DKD) is a sub-type of CKD observed in people with diabetes and a key aspect that should be prioritised alongside other cardiovascular (CV) risk factors during annual type 2 diabetes clinical reviews.
What is DKD?
DKD is a progressive condition strongly associated with adverse CV and renal outcomes, including acute kidney injury (AKI), heart disease and mortality (Afkarian et al, 2013; Winocour, 2017). A small but significant percentage of people may progress to ESKD, requiring renal replacement therapy (dialysis or kidney transplantation; Bilous, 2016).
The classical description of diabetic nephropathy (hyperglycaemia-induced glomerular disease) comprises a pattern of gradually rising urine albumin:creatinine ratio (UACR) followed by a steady reduction in estimated glomerular filtration rate (eGFR; Lewis, 2019). Other microvascular complications are usually present (e.g. retinopathy; Lewis, 2019). However, hypertension and renal atheromatous disease often coexist in diabetes and can cause varying degrees of glomerular damage, tubular fibrosis and interstitial fibrosis (Lewis, 2019). CKD can also arise from causes other than diabetes and vascular disease (Kim et al, 2018), so diabetes and CKD may coexist with no causal link (although this is rare). For practical purposes, people with type 2 diabetes and CKD can be classified as having DKD when no other basis for CKD is identified.
What are the implications of DKD?
Morbidity risk is significantly increased in people with DKD and may be comparable to coronary heart disease (Tziomalos and Athyros, 2015). DKD also represents a substantial economic burden; projections from a recent study utilising the 2010 Health Survey for England predict the economic burden of CKD per million individuals with diabetes to be approximately £11.4 billion by 2025 (Nguyen et al, 2018).
NICE recognises that treatment may prevent or delay the progression of DKD, reduce the onset of complications and lower the risk of CV disease (Heerspink and de Zeeuw, 2011; NICE, 2014). However, early intervention requires timely diagnosis, and screening for DKD plays a critical role in slowing disease progression (Bilous, 2016).
Detecting DKD in people with type 2 diabetes
In the early stages of DKD, there are typically no symptoms. However, early disease can be detected through regular screening. Kidney filtration function is measured using a blood test that assesses eGFR. A urinary albumin test (expressed as UACR) assesses glomerular albumin excretion. When used together, eGFR and UACR testing improve risk stratification and diagnostic accuracy (NICE, 2014).
Decreasing eGFR and a persistently elevated UACR are independent risk factors with similar weightings for important adverse outcomes and mortality (NICE, 2014; Fung et al, 2017). The rate of eGFR decline over time indicates disease progression and provides an indicator of prognosis (Perkins et al, 2011; Nojima et al, 2017). Elevated UACR is an early marker for kidney damage and for future risk of progressive disease, irrespective of the eGFR at the time of measurement (National Kidney Foundation, 2020; Heerspink et al, 2019). The risk of adverse cardiorenal outcomes is multiplied in the presence of both low eGFR and persistently elevated UACR (e.g. stroke, heart failure, ESKD; NICE, 2014).
eGFR testing
A validated formula is used to calculate an individual’s eGFR based upon their serum creatinine level, age, sex and race (Renal Association, 2017). The majority of UK clinical laboratories use the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation to calculate the eGFR, which is recommended by NICE (2014) and considered more accurate than the Modification of Diet in Renal Disease (MDRD) study equation. However, some laboratories have not yet switched from the MDRD to CKD-EPI equation.
UACR testing
Persistently high UACR (albuminuria), denoted by two positive tests (UACR >3 mg/mmol) over a period of three months or more, indicates the presence of kidney damage (National Kidney Foundation, 2012; Bilous, 2016).
Diagnosing kidney disease in people with type 2 diabetes
National and global guidelines on kidney disease are relevant to DKD screening and should be applied when conducting regular reviews of people with type 2 diabetes (KDIGO, 2012; NICE, 2014). Kidney Disease: Improving Global Outcomes (KDIGO; a global non-profit foundation) and NICE have each published guidance on the evaluation and management of CKD (KDIGO, 2012; NICE, 2014). The Renal Association (RA) updated relevant recommendations in 2017 and the Association of British Clinical Diabetologists (ABCD) alongside the RA also issued guidance on the management of hyperglycaemia in people with diabetes and CKD (Renal Association, 2017; Winocour et al, 2018).
UK and international recommendations for the detection and monitoring of kidney disease
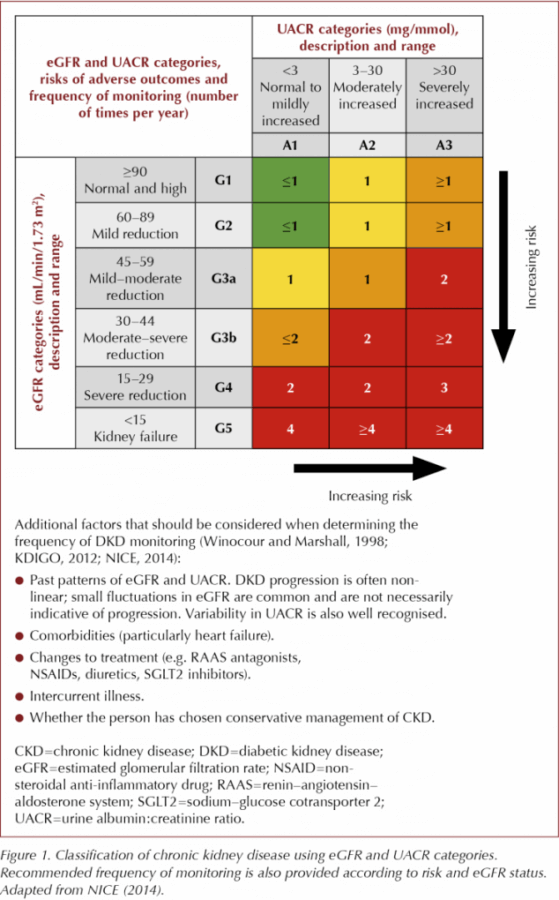
Figure 1 shows the stages of CKD defined by KDIGO (2012), NICE (2014) and the RA (2017). The figure also summarises the recommended frequency of testing and additional factors that should be considered at each stage of disease (Winocour and Marshall, 1998; KDIGO, 2012; NICE, 2014). Following initial diagnosis, individuals are classified as G1–G5 and A1–A3 according to their eGFR and UACR results, respectively.
Initial assessment
Key criteria indicating a diagnosis of DKD are shown below (KDIGO, 2012; NICE, 2014; Renal Association, 2017):
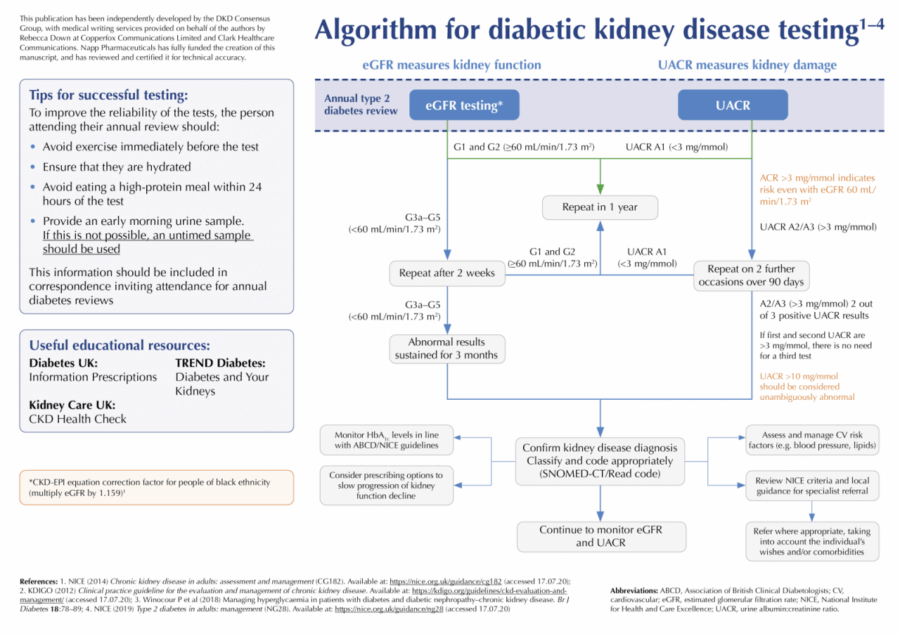
- eGFR <60 mL/min/1.73 m2 on at least two occasions, separated by a period of at least 90 days.
- UACR >3 mg/mmol – when <70 mg/mmol confirmed by repeat UACR – best done using an early morning urine (EMU) sample.
DKD should not be diagnosed on the basis of a single test result. If a new eGFR <60 mL/min/1.73 m2 is identified, repeat eGFR testing is recommended within two weeks to exclude acute kidney injury (AKI; NICE, 2014). Where eGFR is >60 mL/min/1.73 m2, kidney disease should only be diagnosed if another finding is present (NICE, 2014; Renal Association, 2017):
- UACR >3 mg/mmol.
- Haematuria with presumed/confirmed renal cause.
- Electrolyte abnormalities due to tubular disorders.
- Renal histological abnormalities.
- Structural abnormalities (e.g. polycystic kidneys).
- Reflux nephrology.
- History of kidney transplantation.
Frequency of testing/ongoing monitoring
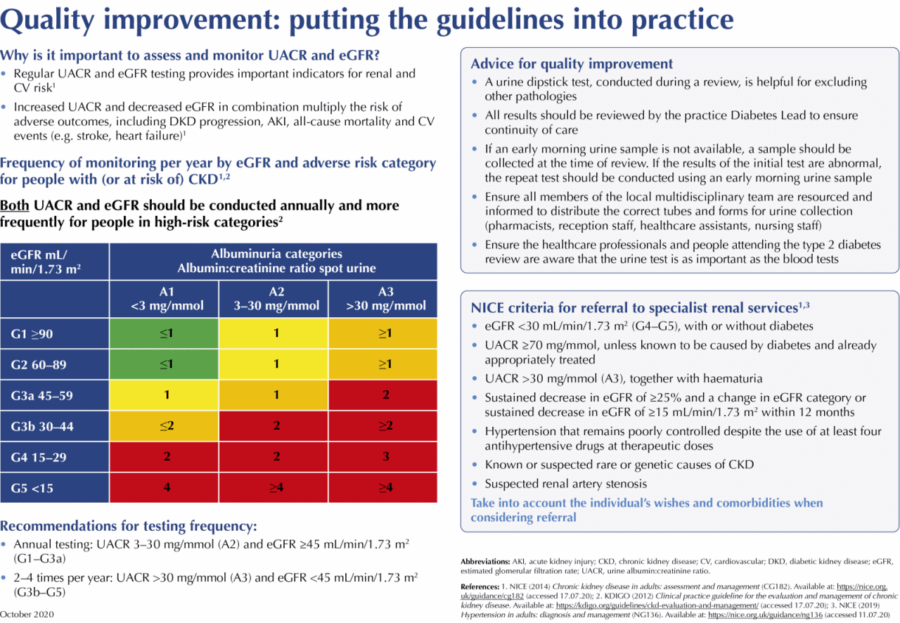
NICE (2014) provides clear recommendations regarding the frequency of eGFR testing for each adverse risk category, although directions for UACR testing are less clear. Given that increased UACR correlates directly with both CV and renal risk, the DKD Testing Consensus Committee agrees with KDIGO (2012) recommendations on this topic, which suggest that UACR and eGFR should both be conducted at least annually for individuals in lower-risk categories (A1–A2 and G1–G3a) and more frequently for those in higher-risk groups (A3 and G3b–G5), as shown in Figure 1.
Identifying progression
Current guidelines define markers for DKD progression as a sustained eGFR decrease ≥25% and a change in eGFR category (G1–G5) within 12 months (KDIGO, 2012; NICE, 2014; Renal Association, 2017). NICE (2014) and the RA (2017) state that a sustained eGFR decrease of ≥15 mL/min/1.73 m2 in one year is indicative of progression. Although KDIGO (2012) defines rapid progression of disease as a sustained eGFR decrease of ≥5 mL/min/1.73 m2 from baseline in 1 year, this magnitude of variation is often seen in clinical practice without cause for concern. The DKD Testing Consensus Committee suggest, therefore, that a drop of 10 mL/min/1.73 m2 may be more appropriate as an indication of progression in those with eGFR >60 mL/min/1.73 m2 (including those with eGFR >90 mL/min/1.73 m2).
If a decline in eGFR is noted, testing should be repeated within two weeks to ensure that the decline is not due to AKI and then again within 3 months (e.g. 4–8 weeks later) to confirm the new CKD stage and exclude continued rapid decline (NICE, 2014).
In addition, the DKD Testing Consensus Committee suggests that increasing UACR levels over time are important in identifying those at greatest risk of CV complications and progression of DKD.
Current testing – what is actually being achieved in practice?
Data from the National CKD Audit show that approximately 86% of people with diabetes receive annual blood tests for DKD, but only 54% have the relevant annual urine tests (Nitsch et al, 2017). Practice variation in the percentage of people with diabetes who were at risk of CKD (but not on the CKD 3–5 Register) and receiving the recommended UACR testing ranged between 0% and approximately 95% (Nitsch et al, 2017). National Diabetes Audit (NDA) data reveal that urine albumin tests are completed less frequently in general practice when compared with other key diabetes care processes (NHS Digital, 2019).
Barriers to testing
The DKD Testing Consensus Committee believes that removal of UACR testing from the Quality Outcomes Framework (QOF) incentive scheme might be responsible for observed reductions in UACR testing across England (NHS Digital, 2019; NHS England, 2019). This may have driven a perception that UACR testing is less valuable in the management of DKD, compared with eGFR testing.
DKD Testing Consensus Committee recommendations for clinical practice
The Committee has agreed on the following consensus recommendations for best practice DKD testing. The DKD Screening/Monitoring Tool is included as supplementary material, which aims to provide a practical quick-reference guide for healthcare professionals.
Initial diagnosis and categorisation of DKD severity are based upon an assessment of both eGFR and UACR, with each test providing an independent measure for risk of adverse outcomes (NICE, 2014). UACR and eGFR should be conducted at least annually for people with type 2 diabetes. Those in higher risk DKD categories should undergo screening more frequently:
- Annual testing: UACR 3–30 mg/mmol (A2) and eGFR ≥45 mL/min/1.73 m2 (G1–G3a).
- 2–4 times per year: UACR >30 mg/mmol (A3) and eGFR <45 mL/min/1.73 m2 (G3b–G5).
eGFR specimen requirements
Clinicians should advise individuals to arrive well-hydrated for their blood test and avoid eating high-protein meals (e.g. steak, hamburger) during the 12 hours prior to their appointment (NICE, 2014). Ensure that blood samples are received and processed within 12 hours of venepuncture.
eGFR pitfalls and cautions
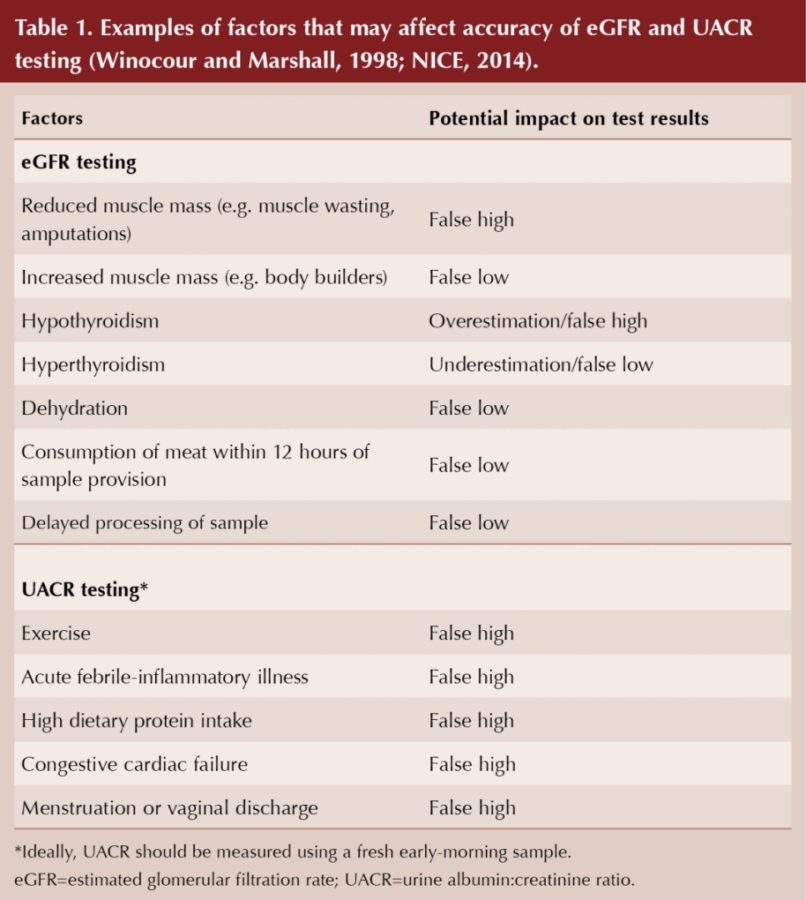
It should be noted that eGFR calculation is an estimate, and a number of factors may affect the accuracy of the result, as summarised in Table 1 (NICE, 2014). The CKD-EPI formula for eGFR under-estimates the true eGFR in obesity and type 2 diabetes (Camargo et al, 2011; Bouquengneau et al, 2013).
Calculations for eGFR assume that the level of creatinine in the blood is consistent (at steady state) and results are invalid in circumstances associated with fluctuating levels (e.g. AKI, dialysis; NICE, 2014; Renal Association, 2017). MDRD and CKD-EPI equations are invalid for individuals under 18 years of age (Renal Association, 2017). There are also pitfalls in over-diagnosing CKD in people over the age of 75 with an eGFR consistent with stage 3 CKD (Ellam et al, 2016) and when there is no other evidence of kidney disease.
Reporting and interpreting eGFR values
Sustained eGFR decline (e.g. >10 mL/min/1.73 m2 over 1 year) should act as a flag for concern and must be raised with the local/practice diabetes lead (Winocour et al, 2020). In people of black ethnicity, apply a correction factor to eGFR values estimated using the CKD-EPI creatinine equation (multiply eGFR by 1.159; NICE, 2014). Allow variability of at least ±5% when interpreting eGFR results (NICE, 2014). In cases where eGFR is <60 mL/min/1.73 m2, confirm the result with repeat testing (within two weeks) to exclude causes of acute deterioration of eGFR (e.g. AKI; NICE, 2014).
Interpret eGFR values ≥60 mL/min/1.73 m2 with caution, as the accuracy of eGFR is reduced above this level (NICE, 2014). An increase in serum creatinine concentration >20% suggests decreased kidney function in people with eGFR >90 mL/min/1.73 m2 (NICE, 2014).
Where AKI is suspected, follow NICE clinical guideline NG148 (NICE, 2019a).
Rationale for UACR testing
UACR is the recommended method for the identification and monitoring of kidney damage in people with diabetes (NICE, 2014). UACR is a more accurate measure than protein:creatinine ratio (PCR), and can help in understanding the underlying causes of renal decline (NICE, 2014; Renal Association, 2017).
UACR specimen requirements
EMU (first void) specimens are the ideal. Use plain collection tubes (usually white top). Tubes containing boric acid (typically red top) are not suitable for UACR specimens. The DKD Testing Consensus Committee recommends that all members of the local multidisciplinary team are resourced and informed to distribute the correct bottles and forms for urine collection (pharmacists, reception staff, healthcare assistants, nursing staff).
Although a first void EMU sample is preferred, a specimen collected at any time of day may be used where a timed sample is not available. Confirm true albuminuria using an EMU specimen when abnormal UACR results are obtained with a random untimed sample (KDIGO, 2012). Ensure that people with type 2 diabetes are informed that they should not collect UACR specimens during an acute illness or menstruation.
UACR pitfalls and cautions
UACR has marked variability, making confirmatory repeat tests important in the diagnosis and monitoring of DKD (Winocour and Marshall, 1998). UACR may be artificially high if the sample is taken under certain conditions (e.g. after exercise), as shown in Table 1 (Winocour and Marshall, 1998). Random untimed urine specimens have an increased risk of false positive results.
Reporting and interpreting UACR values
Normal UACR (<3 mg/mmol) should be coded accordingly and monitored annually (NICE, 2014). UACR between 3–30 mg/mmol is clinically important and must be confirmed using an EMU sample (KDIGO, 2012; NICE, 2014), given the variability of UACR values. However, UACR >10 mg/mmol should be considered unambiguously abnormal.
Individuals with UACR >70 mg/mmol do not require a repeat sample. Unless this increase is known to be caused by diabetes and already appropriately treated (e.g. optimisation of glycaemic control), refer these individuals to nephrology services (NICE, 2014). For nephrotic syndrome (ACR ≥220 mg/mmol) with a low serum albumin, an urgent renal referral is indicated (NICE, 2014).
Other testing considerations
Use dipsticks/reagent strips
NICE and the RA recognise the usefulness of reagent strips as a screening tool for haematuria, but query their sensitivity in the accurate detection of albuminuria, and KDIGO recommends that reagent strip results should be confirmed by clinical laboratory assessment (KDIGO, 2012; NICE, 2014; Renal Association, 2017).
The DKD Testing Consensus Committee believes that it is good practice to perform dipstick/reagent strip analysis for haematuria as an initial assessment at annual type 2 diabetes reviews and whenever there is a significant rise in UACR results. Reagent strip urinalysis can opportunistically reveal other findings, such as leukocytes and/or nitrites, which suggest the presence of a urinary tract infection (UTI) and protein. If protein is detected, the sample should still be sent for UACR, even if a UTI is suspected.
Assessment of haematuria
Causes of haematuria are shown in Box 1 alongside factors associated with urine discolouration or false positive dipstick results. Refer people in appropriate age groups with persistent invisible haematuria (with/without proteinuria) for investigation for urinary tract malignancy (NICE, 2014; 2015). Painless macroscopic/visible haematuria must be urgently referred to urology, unless a definite cause for the bleeding is known (NICE, 2015).
Assessment of CKD progression
A recommendation of KDIGO and NICE is that risk stratification tools can be used for clinical practice. The Kidney Failure Risk Equation uses eGFR and UACR to define the risk of progression of CKD to end-stage renal failure. The tool can be used as an adjunct to testing in routine clinical practice and may be used for prognostic purposes (Major et al, 2019).
Management of DKD
In cases where abnormal eGFR and UACR results persist, the DKD diagnosis must be SNOMED-CT/Read coded appropriately, and the person should be informed of their diagnosis. Aim to reduce CV risk factors through optimisation of glycaemic control, blood pressure and lipid management (NICE, 2014; Dasgupta et al, 2017; Mark et al, 2017; Winocour et al, 2018). Current guidelines recommend the optimal use of evidence-based renoprotective classes of therapy (Dasgupta et al, 2017; Davies et al, 2018).
Education for those with DKD is critical, and materials provided by organisations such as Diabetes UK (e.g. Information Prescriptions) and Kidney Care UK (e.g. CKD Health Check) are helpful and freely available online to support healthcare professionals and people with DKD (Kidney Care UK, 2017; Diabetes UK, 2020).
Referral criteria
Many people with DKD can be optimally managed in primary care. However, where appropriate and in line with local guidance, referral to specialist services may be required. The wishes of the individual and their comorbidities must be considered when assessing suitability for a referral. Box 2 summarises the NICE (2014) criteria for specialist referral in people with kidney disease.
Driving quality improvement in clinical practice
The recommendations outlined by the DKD Testing Consensus Committee aim to enhance the quality of testing and care for people with type 2 diabetes who are at risk of developing or sustaining progression of DKD. It is important that healthcare professionals and people with type 2 diabetes recognise the equal significance of both UACR and eGFR testing in understanding and managing cardiorenal risk in DKD. These tests should be used to inform and guide clinical practice in line with the standards defined by national guidelines and will enable more individualised and effective approaches to DKD management.
Acknowledgements
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
The DKD Testing Consensus Committee would like to thank Alan Jardine (Renal Association) for his contribution to the expert panel meeting, which informed the development of this article.
This publication has been independently developed by the DKD Consensus Group, with medical writing services provided on behalf of the authors by Rebecca Down at Copperfox Communications Limited and Clark Healthcare Communications. Napp Pharmaceuticals has fully funded the creation of this manuscript, and has reviewed and certified it for technical accuracy.
Declarations
Dr Peter H Winocour has received honoraria for delivering educational meetings and/or attending advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Sanofi, Napp Pharmaceuticals, Novo Nordisk and Vifor Pharmaceuticals. He does not hold any shares in any pharmaceutical company.
Jane Diggle is co-Vice-Chair of the Primary Care Diabetes Society (PCDS) and Associate Editor-in-Chief for Diabetes & Primary Care. She has received funding from the following companies for provision of educational sessions and documents, and for attending advisory boards: Abbott, BD, B Braun, Boehringer Ingelheim, BMS, AstraZeneca, Eli Lilly, GlucoRx, Janssen, MSD, Napp Pharmaceuticals, Novo Nordisk, Owen Mumford, Sanofi and Takeda.
Dr Sarah Davies has received speaker honoraria or conference sponsorship from AstraZeneca, Boehringer Ingelheim, Lilly, MSD, Napp Pharmaceuticals, Novo Nordisk and Takeda. She has received advisory board honoraria from AstraZeneca, Novo Nordisk and Takeda. She does not hold any pharmaceutical shares.
Hannah Beba has received speaker honoraria or conference sponsorship from AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Napp Pharmaceuticals, Mylan, Novo Nordisk, Sanofi and Takeda. She has received advisory board honoraria from Novo Nordisk, Napp, Mylan and Takeda. She does not hold any pharmaceutical shares.
Julie Brake has received speaker and advisory board honoraria from Abbott, Astellas, AstraZeneca, Janssen, Lilly, MSD, Napp Pharmaceuticals and Novo Nordisk. She has also received conference registration and subsistence from Janssen, Napp Pharmaceuticals and Novo Nordisk.
Debbie Hicks has received speaker honoraria from Roche, Sanofi and Takeda. She has received speaker and advisory board honoraria from Abbott, AstraZeneca, B. Braun, BD, Boehringer Ingelheim, Gendius, GlucoRx, Janssen, LifeScan, Lilly, MSD, Mylan, Napp Pharmaceuticals, Novo Nordisk, Owen Mumford. Educational sponsorship has been provided by BD, Boehringer Ingelheim, Lilly, MSD, Mylan, Novo Nordisk, Roche, Sanofi and Takeda. She has also received conference registration and subsistence from BD, Lilly and Novo Nordisk. Debbie does not hold any shares in any pharmaceutical company.
Paul Cockwell has received honoraria for speaking or preparing non-promotional educational material from Janssen Pharmaceuticals, Merck Sharp & Dohme Ltd and Napp Pharmaceuticals. He does not hold any shares in any pharmaceutical company.
Claire Main has no financial disclosures.
Consensus Committee
The DKD Testing Consensus Committee comprises a panel of clinical experts from across primary and specialist care and pharmacy services, who met with the objective of supporting quality improvement in DKD screening, diagnosis and ongoing monitoring. The Committee’s goal was to provide healthcare professionals with comprehensive but succinct and practical guidance to support DKD testing in people with type 2 diabetes.
Endorsements
This document and the supplementary material have been endorsed by: Primary Care Diabetes Society (PCDS); Association of Clinical Diabetologists (ABCD); UK Clinical Pharmacy Association; TREND Diabetes; Renal Association; Association of Nephrology Nurses; and Diabetes UK (DUK).






Jane Diggle discusses emotional health and diabetes distress, and offers some tips for discussing this in our consultations.
11 Nov 2025