If we were told that there was a medical condition that affected up to 25% of the adult population (Romero-Corral et al, 2010), significantly increased the risk of major adverse cardiac events (Xie et al, 2017), caused secondary hypertension (Dopp et al, 2007), impacted glucose intolerance and insulin resistance (IDF, 2008), affected 23% of diabetes patients (IDF, 2008), significantly increased the risk of erectile dysfunction (Teloken et al, 2006) and was responsible for 15–20% of reported road traffic accidents (Findley and Stuart, 2001), we would surely consider it to be one of the most important medical problems we face in society today. This condition does exist, however, and it is called obstructive sleep apnoea (OSA). All of these facts lead to the questions: What is OSA? Who is at risk? And should OSA be diagnosed in our primary care diabetes clinics?
What OSA is and how to identify it
OSA is the most common form of sleep-disordered breathing (SDB). Many people use the terms SDB and OSA interchangeably, but SDB includes:
- OSA
- Central sleep apnoea
- Cheyne-Stokes respiration
- Nocturnal hypoventilation.
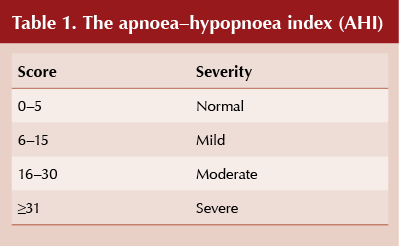
The term apnoea relates to a 0–20% reduction in airflow lasting ≥10 seconds and hypopnoea to any reduction in airflow lasting ≥10 seconds. OSA is measured by the apnoea–hypopnoea index (AHI; see Table 1), which divides the total number of apnoeas plus hypopnoeas by the number of hours slept to come up with a score. For example, if in 6 hours there are 125 apnoeas and 75 hypopnoeas, the AHI is (125 + 75)/6 = 33.
Possible OSA can be highlighted by asking patients about the “triple S” symptoms:
- Snoring when asleep
- Stopping breathing or Struggling to breathe when asleep
- Sleepy when awake.
Patients can initially be assessed in the primary care setting with the Epworth sleepiness scale, Berlin questionnaire or STOP-BANG questionnaire, which are freely available online. Those with suspected OSA should be referred to secondary care for assessment and treatment. Details of the tools and management options available for OSA will be covered in a future issue of this journal.
Prevalence of OSA
OSA affects a large number of people but, worryingly, an estimated eight out of 10 patients are undiagnosed. OSA is more prevalent in males than females, but the actual figures quoted vary depending on the definition of AHI used and the patient cohort. In adults aged 30–60 years with a BMI of 25–28 kg/m2, 24% of men and 9% of women have an AHI >5, and 9% of men and 4% of women have an AHI >15 (Young et al, 2002). In total, approximately one in five adults present with mild and 1 in 15 with moderate or severe OSA (Young et al, 2002). Sleep disorders are also common in the paediatric population, with 25–37% being affected, and OSA being present in 1–4% of children (Rosen, 2017).
Why should we assess patients for OSA?
Research over the past 20 years has shown that OSA is an independent risk factor for a number of other disorders including hypertension, cardiovascular disease, stroke and metabolic dysfunction (IDF, 2008). Diagnosing and treating OSA has therefore become a major clinical consideration.
There is a strong correlation between OSA and cardiovascular disease, with an estimated 38 000 cardiovascular deaths occurring each year in people with untreated sleep apnoea (Young et al, 2002). Improvements in cardiovascular disease have been demonstrated with appropriate treatment of OSA: a 10-year follow-up study found that participants with untreated OSA had a higher risk of both fatal and non-fatal cardiovascular events than those whose OSA was treated (Marin et al, 2005).
Undiagnosed sleep apnoea is also independently associated with an increase in the incidence of hypertension (Young et al, 2002). One in three people with hypertension have OSA and 80% of people with drug-resistant hypertension have OSA (Young et al, 2002).
In addition, there is an association between OSA and impaired glucose tolerance. People with appropriately-treated OSA (continuous positive airway pressure for >4 hours per night) have significantly better glucose tolerance than those who are not treated (Babu et al, 2005). There is a recognised association between OSA and type 2 diabetes, but its exact nature has yet to be fully elucidated (International Diabetes Federation, 2008).
OSA is not just associated with comorbidities, however, but with mortality itself. In the Wisconsin Sleep Study, which followed 1522 participants over 18 years, untreated OSA resulted in a nearly four-fold increase in all-cause mortality and a five-fold increase in cardiovascular mortality (Young et al, 2008).
National initiatives
There are several initiatives that are attempting to increase awareness of and knowledge about OSA.
The OSA Partnership Group, which includes representatives from the commercial vehicle sector, clinicians, patient groups and those interested in health and safety at work, is campaigning to raise awareness of OSA and its consequences. As a result of its work, the DVLA has changed the wording in the Assessing Fitness to Drive for medical professionals in order to clarify the driving regulations for people with OSA. The focus is now on excessive sleepiness having, or likely to have, an adverse effect on driving, rather than on AHI. The DVLA has also updated the SL1 (medical questionnaire), which will soon be on the Gov.uk website. The British Thoracic Society (2018) has launched an updated position statement on Driving and Obstructive Sleep Apnoea following the DVLA changes.
A NICE guideline on sleep-disordered breathing is currently in development and is due to be published in August 2020. It is hoped that the guideline will advocate the 4-week wait/fast-tracking for vocational drivers suspected of having or diagnosed with OSA.
In addition to this, in 2017 the World Association of Sleep Medicine and World Sleep Federation launched the National Sleep Day initiative (http://worldsleepday.org) to raise awareness of sleep-related issues. The initiative aims to advance knowledge about sleep, circadian rhythms, sleep health and sleep disorders, and to promote sleep health by advancing public education, supporting related public policies and research.
Assess your diabetes patients!
Given the seriousness of OSA, we should be screening for the condition in our routine diabetes clinics and referring possible cases to secondary care for assessment and management. Mild OSA can be effectively treated with behavioural modification and moderate to severe OSA with continuous positive airway pressure (CPAP). In the long-term, we should be looking to provide screening, diagnosis and even treatment of OSA in a primary care setting.





Risk ratios of 1.25 for autism spectrum disorder and 1.30 for ADHD observed in offspring of mothers with diabetes in pregnancy.
18 Jun 2025