It has been estimated that 700 million people globally will have diabetes by the year 2045 (Saeedi et al, 2019). Diabetic sensorimotor peripheral neuropathy (DSPN) is a complication of diabetes and is the main precipitating factor for diabetic foot complications (Sloan et al, 2021). The prevalence of painful DSPN has been estimated to be between 13% and 35% (Ponirakis et al, 2019). This article briefly outlines the epidemiology and pathophysiology of painful DSPN, focusing on current medical management, and aims to highlight the evidence and underutilised role of spinal cord stimulation (SCS) in the management of painful DSPN.
Painful DSPN – risk factors and epidemiology
Risk factors for painful DSPN are poorly understood and associations have been reported with the duration of diabetes, HbA1c and body weight (Raputova et al, 2017). Meng and colleagues (2015) found genetic alterations associated with neuronal excitability in individuals suffering with painful DSPN. Painful DSPN impacts significantly on quality of life, and symptoms include a burning type of pain, electric shock-type pains and a cold, aching pain. People with DSPN report increased pain with a painful stimulus (hyperalgesia) as well as pain due to stimuli that do not normally provoke pain (allodynia). Pain is typically worse at night and some individuals report pain on weight-bearing. Peripheral neuropathic pain is known to have a significant impact on health-related quality of life, and individuals with painful DSPN are often profoundly disabled. Chronic pain results in low mood as a consequence of the impact that pain has on an individual’s ability to function, resulting in a reduced quality of life and an inability to maintain employment (Jensen et al, 2007).
Pathophysiological mechanisms of painful DSPN
The pathophysiological mechanisms of the DSPN are multifactorial. Peripheral nerves require an adequate blood supply, intact glucose and lipid metabolism, and mitochondrial function for their optimal function, all of which can be disturbed in diabetes (Sloan et al, 2021). Animal models are generally used to study the pathophysiology of neuropathic pain, including DSPN. However, no model adequately mimics the human condition, which has hampered the translation of successful treatments from animal models.
Neuropathic pain is defined as pain due to an injury or disease of the peripheral or central nervous system, and is characterised by spontaneous and evoked (due to contact or movement) types of pain, which are underpinned by various distinct changes in the structure of the peripheral and central nervous systems.
In some individuals, the nerve lesion triggers molecular changes in nociceptive neurons (nerves that sense harm), which become abnormally sensitive and develop pathological, spontaneous activity. Inflammatory reactions of the damaged nerve trunk can induce ectopic nociceptor activity, causing spontaneous pain. The hyperactivity in nociceptors induces secondary changes in processing neurons in the spinal cord and brain, so that input from mechanoreceptive A-fibres (light touch and vibration, and position sense) is perceived as pain. Neuroplastic changes in the central pain modulatory systems (descending and ascending pain pathways) can lead to further hyperexcitability.
What is poorly understood is why some individuals with DSPN develop painful symptoms, whilst others are largely asymptomatic. It has, therefore, been suggested that, as with other chronic pain syndromes, an interaction between biological, psychological and social factors causes changes within the central and peripheral nervous system that result in the development of chronic peripheral neuropathic pain. These factors include environmental and genetic factors, and vascular and metabolic abnormalities. Individuals with painful DSPN display small fibre peripheral neuropathy, as well as autonomic dysfunction and vascular alterations. Inflammation of the nervous system leads to neuronal hypersensitivity, and dysfunction of microglia may contribute to neuropathic pain. Increasing signals from damaged peripheral nerves arriving at the dorsal horn of the spinal cord lead to an increase in spontaneous activity at the level of the spinal cord, with activation of normally quiescent receptors. For example, activation of N-methyl-d-aspartate (NMDA) receptors, which are normally blocked by magnesium, results in an enhanced and amplified transmission of neural activity from the spinal cord to the brain. Changes within brain areas, such as higher pain centres and thalamus, also result in the maintenance of persistent pain. Morley and Williams (2015) explain that excitatory pain pathways become active and inhibitory pathways become quiet in the presence of anxiety, depression, threat and catastrophic thoughts about pain. The opposite happens as people with pain learn to regain control by various methods, such as cognitive behavioural therapy or mindfulness.
Management of individuals who suffer with painful DSPN
The biopsychosocial model of chronic pain management
The biopsychosocial model of pain proposes that biological factors can influence physiological changes, and that psychological factors are reflected in the appraisal and perception of internal physiological phenomena (the abnormal sensations generated by damaged nerves, for example). These appraisals and behavioural responses are, in turn, influenced by social or environmental factors. At the same time, the model also proposes that psychological and social factors can influence biological factors, such as hormone production, activity in the autonomic nervous system and physical deconditioning (Gatchel et al, 2007). The management of chronic pain, therefore, requires the use of a biopsychosocial approach that appreciates that persistent pain is a disease rather than merely a symptom, an idea that has been encapsulated in the new IASP classification of chronic pain as part of the International Classification of Diseases (ICD-11; Treede et al, 2019).
The management of painful DSPN should have as its foundation a biopsychosocial approach which, by necessity, requires an interdisciplinary team. The management of an individual with DSPN might include engagement with an endocrinologist, neurologist, psychologist/podiatrist pain specialist, orthopaedic surgeon, vascular surgeon and microbiologist. In the past, pain clinics in the UK have managed individuals with painful DSPN. However, the establishment of protocols within primary care has meant that we do not generally see individuals with this condition in pain services unless local relationships with diabetes services are established. Symptoms that should be addressed include the individual’s pain together with associated mood disorders, such as depression and anxiety, and the consequences of loss of sensation and motor power, which might include unsteadiness and falls, as well as autonomic symptoms.
Diagnosis
The Toronto Diabetic Neuropathy Expert Group has established a consensus definition of DSPN. Individuals with diabetes are largely managed in primary care and the diagnosis of DSPN often falls to diabetes nurses. Bedside examination of the feet and legs, including assessment of temperature or pinprick sensation, may be used. A number of instruments have been employed to assess neuropathic pain, including the painDETECT tool and DN4 questionnaire. A careful clinical history should exclude other causes of neuropathy unrelated to diabetes, as per the NeuPSIG guidelines for the diagnosis of neuropathic pain (Finnerup et al, 2016).
Medication
Individuals who present with painful diabetic peripheral neuropathy are usually commenced on medication in the form of antidepressant and antiepileptic medications, as per national guidance in the UK. Referral to a secondary diabetes service should be triggered when individuals fail to respond to initial management.
The treatments for painful DSPN are purely symptomatic and do not alter the disease processes. The number needed to treat for around 50% pain relief ranges from 4 to 10. Often, however, individuals experience significant side-effects to these medications.
Antiepileptic medication
Medications that act on the alpha-2-delta ligand of calcium channels within the spinal cord are the most robust in terms of evidence in the management of painful DSPN. Pregabalin and gabapentin are non-selective ligands for subunits of voltage-gated calcium channels and reduce neuronal hyperexcitability at the level of the spinal cord. Gabapentin, at doses of more than 1200 mg/day, has been shown to have efficacy in painful DSPN (Wiffen et al, 2017). Pregabalin is superior to placebo at doses of 300 and 600 mg/day. Common adverse effects of these medications include dizziness, somnolence, euphoria, peripheral oedema and weight gain.
Tricyclic antidepressants
Amitriptyline is the most frequently prescribed medication. Adverse effects of tricyclic antidepressants include postural hypotension, urinary retention, dry mouth and dizziness.
Serotonergic–noradrenergic reuptake inhibitors (SNRIs)
Duloxetine is the most prescribed SNRI for painful DSPN. Doses of 60 to 120 mg/day significantly reduce the severity of neuropathic pain. Venlafaxine is an alternative agent, although there is less evidence to support its use (Lunn et al, 2014).
Combination therapy
There is little evidence to support the approach of using antidepressant and anticonvulsant medications in combination. Tesfaye et al (2013) compared pregabalin and duloxetine at moderate doses against high-dose monotherapy, but found no statistically significant superiority with a combined approach.
Opioids
There is increasing evidence that the use of opioids for chronic pain management is associated with high rates of opioid dependence, increased mortality, endocrine dysfunction and compromise of the immune system (Vowles et al, 2015). The evidence base for the management of neuropathic pain with opioids is limited. The use of opioids in the management of neuropathic pain should not be undertaken outside of a specialist setting.
Spinal cord stimulation
SCS involves the insertion of platinum–iridium electrodes into the epidural space and the application of an electrical current to the axons of the spinal cord to produce the release of neurotransmitters and reduce neuronal excitability. Electrical stimulation of the spinal cord was first used in 1967, although electricity has been used to treat pain since antiquity. Advances in SCS technology have evolved alongside pacemaker technology, with improvements in lead construction and implantable pulse-generator performance.
Mechanism of action
The development of SCS arose out of the Gate Control Theory of Pain. Melzack and Wall (1965) postulated that a gate system modulates the sensory input from the skin before it evokes pain perception and response in the brain. Activation of large fibres serving light touch and vibration sensation results in the inhibition of small nerve-fibre activity, which transmits pain. Hyperactive pain-projecting neurons are implicated in chronic pain.
Low-frequency SCS stimulates surface dorsal column fibres, which are linked to inhibitory interneurons. When stimulated, the inhibitory interneurons release the inhibitory neurotransmitter GABA, which acts on the hyperactive pain-projecting neurons (Myerson and Linderoth, 2000). These neurons are then hyperpolarised, which reduces pain signals to the brain. The stimulation of the dorsal columns causes a tingling sensation in the area of pain, which produces pain relief.
Disadvantages of paraesthesia-based stimulation include that when the lead moves relative to the spinal cord, the area of pain is not covered by the tingling sensation produced by the device. The efficacy of the therapy is related to the degree of paraesthesia coverage.
More recently, a paraesthesia-independent mechanism of SCS has been developed in the form of a 10-kHz current (high-frequency SCS) delivered to the spinal cord. This does not require paraesthesia coverage in order to achieve pain relief. It is thought that the application of a 10-kHz current directly inhibits superficial neurons in the dorsal horn of the spinal cord (Bradley et al, 2017). The mechanism of action of high-frequency SCS in the management of peripheral neuropathic pain may be conceptualised as a runaway car that is accelerating out of control (i.e. acceleration is pain due to hyperactivity of the spinal cord) and the application of a high-frequency current to the spinal cord applies the brakes to the car, slowing it down (reduction in spinal cord hyperactivity and, therefore, pain).
Patient selection
SCS is indicated for the management of peripheral neuropathic pain as per NICE guidance (www.nice.org.uk/guidance/ta159) in individuals who continue to report more than 50 mm of pain on a visual analogue scale despite 6 months of conventional medical management.
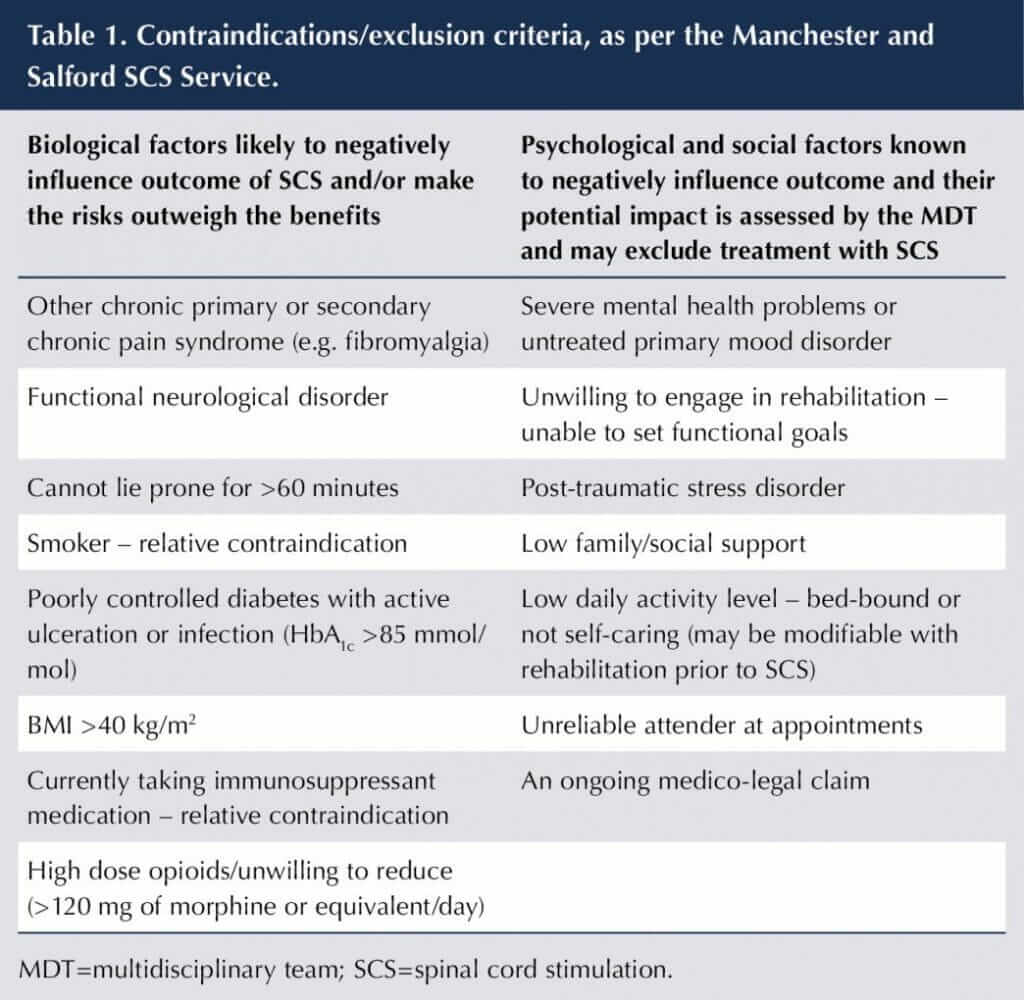
However, there are biological, psychological and social factors that potentially impact on the success of the therapy. Our unit has devised the guidance shown in Table 1 in order to optimise patient selection, recognising the impact of biological, psychological and social factors that influence success with the therapy. SCS inhibits neuronal activity at the level of the spinal cord, thereby reducing the volume of signals reaching the brain. If, however, the signals that reach the brain are interpreted in a catastrophic manner, or by a brain that is biochemically at a disadvantage because of psychological and social stressors, then the reduction in neuronal excitability at the level of the spinal cord does not translate into a reduction in pain and improvement in quality of life. Psychosocial factors are potentially modifiable, and an individual may require physiotherapy and psychology pain management input prior to having a spinal cord stimulator inserted.
Obesity, smoking and high-dose opiates increase the risk of infection of the device. The presence of another chronic pain condition or a functional neurological disorder undermines the effectiveness of SCS. Reduction of the symptoms of neuropathic pain in the presence of another chronic pain syndrome does not result in an improvement in function, because of the impact of the associated neurological disorder or chronic pain syndrome. The inability to lie prone for more than 60 minutes is a practical consideration because insertion of the device under local anaesthesia takes place in this position.
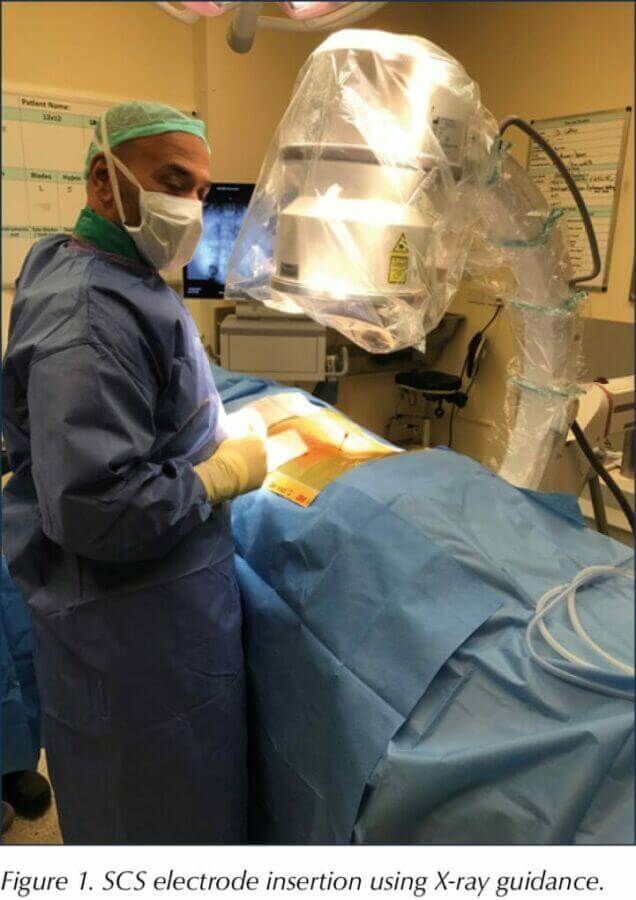
The procedure
The patient is listed for the procedure after a multidisciplinary team assessment, which includes an evaluation by a clinical psychologist and education about living with an SCS system delivered by a specialist neuromodulation nurse. Individuals receive preoperative antibiotics. The procedure is performed in an operating theatre under sterile conditions. Insertion of a spinal cord stimulator implant takes approximately 60 minutes and is conducted as a day-case procedure (see Figures 1 and 2).
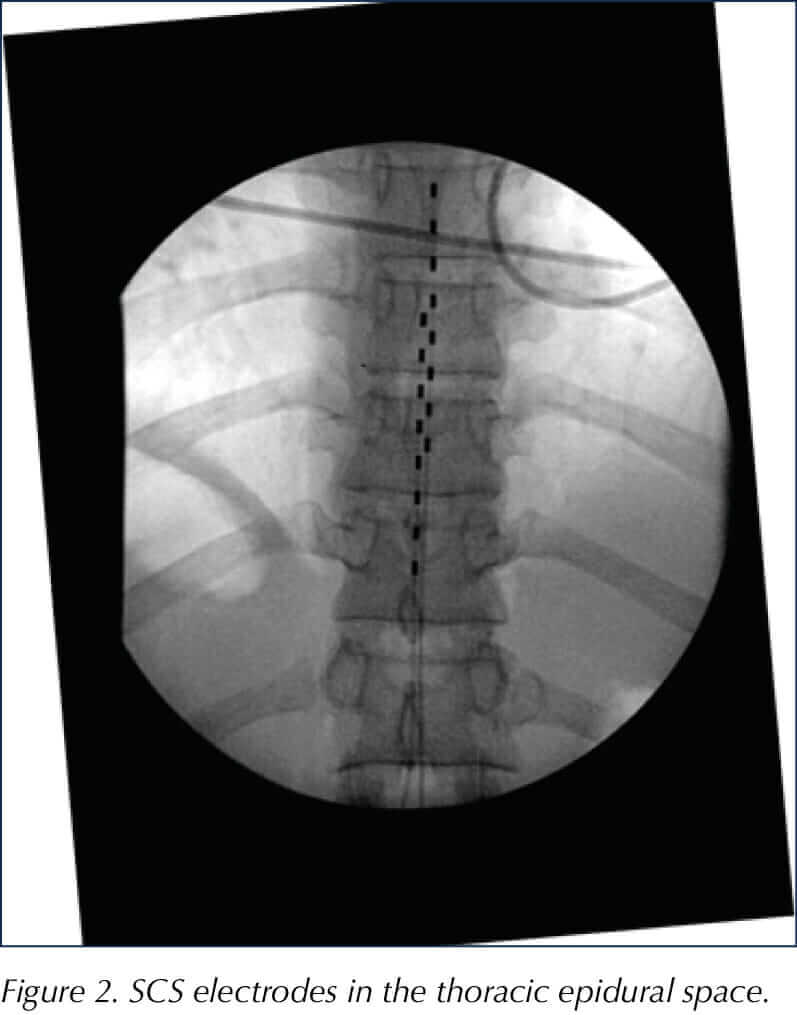
Practice varies between implanting centres. Our practice is to forego the 10-day trial period, where temporary epidural leads are inserted and connected to an external battery. We now insert the complete spinal cord stimulator system, which comprises two leads placed over the thoracic spine between T9 and T11 for pain in the lower limb or in the cervical epidural space for upper-limb pain. The leads are connected to an implantable pulse generator, which is placed just above the patient’s buttocks (see Figure 3).

Evidence of efficacy and effectiveness in painful DSPN
Low-frequency, paraesthesia-based SCS is effective in treating intractable pain associated with many peripheral neuropathies, including painful DSPN. Significant pain relief is achieved in approximately 60% of individuals using conventional (paraesthesia-based) SCS therapy (Tesfaye et al, 1996). Petersen et al (2021) conducted a prospective, multicentre, randomised controlled trial using high-frequency SCS and found that 79% of individuals with refractory painful DSPN achieved more than 50% pain relief, compared to 5% of those assigned to conventional medical management.
Service delivery considerations
The patient population most commonly treated with SCS is those who have persistent pain following technically successful spinal decompressive surgery and who continue to suffer with neuropathic pain owing to injury of lumbar or cervical nerve roots. Access to SCS, however, depends on the neurosurgeon or spinal orthopaedic surgeon and/or the individual’s GP recognising that neuropathic pain should be treated according to NICE guidance, with referral for an assessment for SCS if the individual continues to experience more than 50 mm of pain on a visual analogue scale despite 6 months of conventional medical management. Practically, however, this does not happen consistently within the UK, and many suitable individuals do not have access to the therapy (Vyawahare et al, 2014).
The evidence base for the management of painful DSPN with conventional SCS dates back to 1996. However, the lack of robust referral pathways between diabetes services, in both primary and secondary care, and pain services who implant these devices means that there is inconsistent and inequitable access to SCS. Individuals are then established either on no treatment when anticonvulsant and antidepressant medications fail, continued on these medications despite significant side-effects (with impairment of quality of life) or, even worse, are established on high-dose opiate therapies (with significant psychosocial and biological consequences).
Unless pathways are established between primary and secondary care (particularly in the guidance used by specialist nurses working in diabetes services) and specialised pain services, it is likely that the inequitable situation above will continue, even in light of the recent evidence for the efficacy of high-frequency SCS for the management of painful DSPN. SCS needs to form part of national primary and secondary care guidelines with regards to the management of painful DSPN, with locally implemented pathways between diabetes service networks and the pain services that implant these devices.




Why ACR screening is the key to improving renal outcomes in people with diabetes.
21 Oct 2024