The update to the NICE NG28 guidance on management of type 2 diabetes in adults (NICE, 2015) has been long awaited. The most significant change in this 2022 update is to the recommendations regarding glycaemic control.
Optimal glycaemic management is an acknowledged element of good diabetes care. However, many individuals fail to achieve and maintain their glycaemic targets (Khunti, 2013; Blonde, 2017; Bain, 2020). It is recommended that the target HbA1c level be individualised to account for multiple factors. These factors include:
- The individual’s personal preferences.
- Comorbidities.
- Risks from polypharmacy.
- The likelihood of benefiting from long-term interventions.
In line with the growing evidence base and other national and international guidance (SIGN, 2017; Buse et al, 2020), NG28 now encourages the identification of those with either atherosclerotic cardiovascular disease (ASCVD), chronic heart failure or high risk of CVD. The determination of high cardiovascular risk is defined as:
- A QRISK2 score of >10% in adults aged 40 years and over, or:
- An elevated lifetime risk of CVD (defined as the presence of one or more cardiovascular risk factor in someone under 40 years. These risk factors are hypertension, dyslipidaemia, smoking, obesity and family history [in a first-degree relative] of premature CVD).
This identification process should take place at diagnosis and at every diabetes review thereafter.
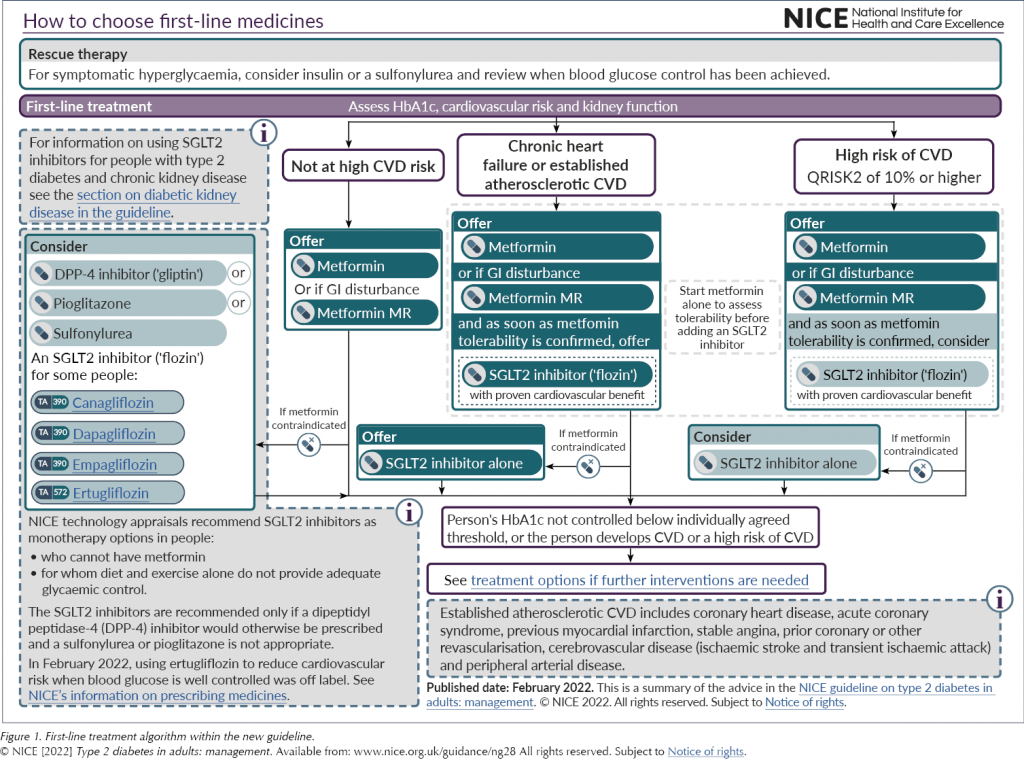
For any patient falling into these three categories, whether initially or at a subsequent review, the advice now is to offer dual therapy with metformin and an SGLT2 inhibitor with proven cardiovascular benefit; metformin is to be started until tolerability is established and then the SGLT2 inhibitor should be started immediately. Where metformin is contraindicated or not tolerated, the SGLT2 inhibitor should be used first-line. This is a real shift in current practice and brings the guidance in line with many other global recommendations.
SGLT2 inhibitors
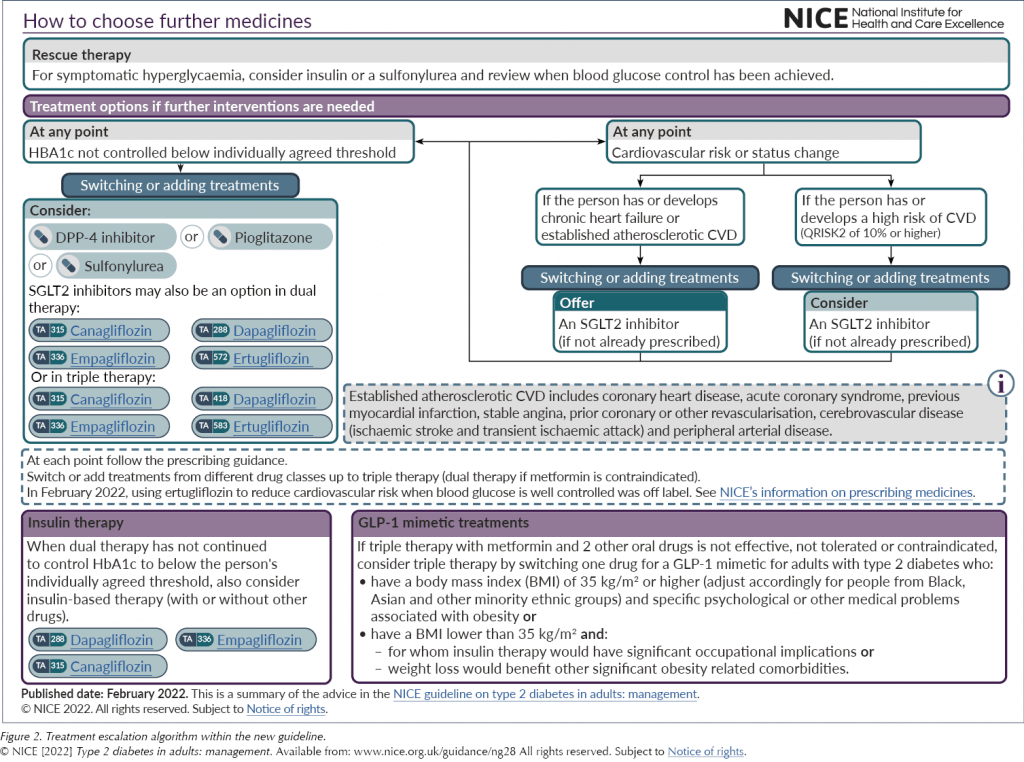
The SGLT2 inhibitor class of medications now has a far greater prominence across the treatment algorithms, both for initial management and if further intensification is indicated (see Figure 1 and Figure 2). This recognises the growing evidence base for the class.
However, as Brown (2022) has pointed out, the recommendation that SGLT2 inhibitors be offered to all people with type 2 diabetes and chronic heart failure does not differentiate between the different types or stability of heart failure, and this could lead to potentially unsafe prescribing of SGLT2 inhibitors outside of their licence. Furthermore, as SGLT2 inhibitors are not currently licensed in the UK for reduction of cardiovascular risk when glucose is at target, people will also need to be counselled about off-licence use.
Renal indications
In addition to the cardiovascular evidence, the guideline also acknowledges the renal benefits now demonstrated in the SGLT2 inhibitor class. Strong evidence from randomised controlled trials clearly shows that these agents reduce the risk of chronic kidney disease (CKD) progression, mortality and cardiovascular events in adults with type 2 diabetes and CKD. NG28 links to the NICE CKD guideline (NG203), which was published recently (NICE, 2021). NG203 recommends offering an SGLT2 inhibitor in addition to an ACEi/ARB in all those with an albumin:creatinine ratio (ACR) >30 mg/mmol. Furthermore, it encourages the consideration of this combination in those with an ACR of 3–30 mg/mmol (depending on individual drug licence indications, noting that not all SGLT2 inhibitors are currently licensed for this use). The addition of an SGLT2 inhibitor in these patients is due to the class’s demonstrated renal benefits, as the ability to lower glucose diminishes with lower renal function.
DKA risk
Prior to starting an SGLT2 inhibitor, the risk of developing diabetic ketoacidosis (DKA) must be assessed. The risk is greater in some patients, especially if:
- They have had a previous episode of DKA.
- They are unwell with intercurrent illness.
The additional recognition of very-low-carbohydrate or ketogenic diets as a risk factor for developing DKA in those taking an SGLT2 inhibitor has been included in this update. The advice in these situations is to delay the introduction of an SGLT2 inhibitor until the diet has been modified. For patients already on an SGLT2 inhibitor, the clear advice is to recommend they do not start a very-low-carbohydrate or ketogenic diet without alerting their healthcare professional first. In these circumstances, the use of the SGLT2 inhibitor may be suspended for the period in which the diet is being followed.
What if an SGLT2 inhibitor cannot be used?
The final point to note in this section on SGLT2 inhibitors is that, for those with established or high risk of CVD who are unable to tolerate an SGLT2 inhibitor, the recommendation is for them to remain on metformin alone. The decision not to recommend a GLP-1 RA at this point is a controversial issue.
The above changes to the guidance are welcomed and regarded by many as long overdue; however, the absence of the GLP-1 RA class early in the treatment algorithm is more contentious.
GLP-1 RAs
The positioning of the GLP-1 RA class of medications is likely to be one of the greatest discussion points for the updated guidance. The decision of NICE to continue positioning the class as a fourth-line option puts the guidance at odds with many of the other guidelines across the world (SIGN, 2017; Buse et al, 2020).
GLP-1 RAs remain as a treatment option only after failure to achieve glycaemic control on three oral therapies, and there has been only one adjustment to the previous advice. Before, the guideline only supported the use of a GLP-1 RA alongside metformin and a sulfonylurea, and not with an SGLT2 inhibitor; this has now been updated to reflect the more prominent position of SGLT2 inhibitors in the treatment pathway as a whole.
Keeping GLP-1 RAs as only a fourth-line option moves this guidance further from the American Diabetes Association (ADA)’s Standards of Care (ADA Professional Practice Committee, 2022). In these standards there is a clear recommendation, much earlier in the pathway, for the use of SGLT2i/GLP-1 RA combination therapy in those with established or high risk of CVD.
Why are the GLP-1 RAs sidelined?
It has been acknowledged that the cardiovascular benefits of the GLP-1 RA class were not reviewed in depth for this update. Meanwhile, given the wide range of options in this class (twice-daily, daily and weekly injectables and an oral preparation) and the variable efficacy between the individual drugs (for more information, see Morris, 2021), the cost-effectiveness for glucose lowering has not been established for the class as a whole, and so the advice has not been altered since the 2015 update. As such, and reviewing the class purely on its glucose-lowering effect, NICE has continued recommending a BMI cut-off for starting a GLP-1 RA of ≥35 kg/m2, unless insulin therapy would have significant occupational implications or weight loss would improve other significant obesity-related comorbidities.
It also remains the case that the GLP-1 RA must be stopped if both HbA1c and weight have not significantly reduced (by 11 mmol/mol and 3% of body weight, respectively) at 6 months. Whilst it is always appropriate to review and stop medications where there has been no clear benefit, achieving both weight and HbA1c reductions can be challenging.
A further review of the cardiovascular benefits of GLP-1 RAs will begin in April 2022, and we will have to wait for this review to complete before any change to the positioning of the GLP-1 RA class can be expected.
Other oral medications
Very little has changed for the other oral medication classes in terms of recommendations for initial or follow-on therapy. They remain options in dual or triple therapy. In addition, they can be offered as first-line therapy in the following circumstances.
Sulfonylureas
In addition to being used as a rescue therapy option in people who are osmotically symptomatic, sulfonylureas are recommended as an option for individuals who are unable to take metformin due to either contraindication or intolerability, if they are not identified as being in the ASCVD, heart failure or CKD risk groups (in which case an SGLT2 inhibitor should be offered).
DPP-4 inhibitors
Like sulfonylureas, this class of medication is recommended for patients as a first-line option for those who are unable to take metformin either due to contraindication or intolerability. A DPP-4 inhibitor can be considered if patients are not identified as being in the ASCVD, heart failure or CKD risk groups.
Pioglitazone
Again, like sulfonylureas and DPP-4 inhibitors, pioglitazone (the only remaining licensed TZD in the class) is recommended as a first-line option for patients who are unable to take metformin either due to contraindication or intolerability, if they are not identified as being in the ASCVD, HF or CKD risk factor groups.
Insulin
Very little has changed in the advice on insulin. As well as being a third- or fourth-line option for glycaemic control, it remains an option in rescue therapy, like sulfonylureas, to normalise glycaemia quickly to eradicate osmotic symptoms.
The emphasis for choosing a biosimilar insulin, if available, is supported in this update. More information on biosimilars can be found in this journal (Morris, 2022).
Other updated sections
Individualised care
The importance of individualising care is emphasised. To further support the individualisation of glycaemic targets, a patient decision aid has been produced. This also encourages the relaxation of targets in those with frailty.

Diagnosing and managing hypertension
The management of blood pressure is a key component of diabetes care. The identification and management of hypertension in people with diabetes has been removed from this guidance as they are included in the NG136 guideline Hypertension in adults: diagnosis and management (NICE, 2019). A review of the updated guidance was published in this journal (Milne, 2019).
Conclusions
In summary, there are a number of positive aspects to the updated NG28 guidance. The decision aid tool and recognition of the need to de-escalate therapy in the frail person, as well as the long-awaited shift in position to the early use of SGLT2 inhibitors, are all very welcome. In addition, a review of glucose monitoring has since been published at the end of March 2022. This update extends the option of flash glucose monitoring to certain individuals with type 2 diabetes who are on multiple daily insulin injection regimens, which should prove very useful.
However, there were also some disappointments in the updated guidance. As we still have to wait for a further, wider review of GLP-1 RAs, one could argue that the guideline still feels behind the times in some areas, and that an opportunity is being missed for a potentially useful class of drugs.






Key scientific developments presented at the conference.
6 Aug 2025