The FreeStyle Libre is currently the only flash glucose monitoring system available on the NHS for healthcare professionals to prescribe. It is a small device implanted in the upper arm, which remains in place for 14 days. The sensor constantly measures interstitial fluid glucose levels and collects data. Users can apply a handheld scanner to the sensor to see their current glucose level, glucose data from the last 8 hours and an arrow showing the direction and speed at which their glucose level is heading.
The Libre is also supported by an IT software programme called LibreView, and a smartphone app and companion website called LibreLink, both free to all users. This allows the data collected via the sensors to be uploaded to the Cloud. Data can be shared with healthcare professionals and family members, which means viewing and interpretation can be streamlined and is easier for all involved. The data can be displayed in the form of many different views and graphs.
When the direction of glucose travel is changing quickly (signified by a downward- or upward-facing arrow) the person with diabetes will still need to check glucose levels with a capillary test. Due to the way the Libre measures glucose levels in the interstitial fluid rather than the blood, there can be a slight lag time in the data it shows. For some the delay can be as high as 10–20 minutes, although on average it is 5–7 minutes.
The Libre has replaced the need to finger-prick for some people, although there are exceptions where this is still required. Initially the Driver and Vehicle Licensing Agency (DVLA) did not accept flash glucose monitoring as safe for use when driving; however, this was recently reviewed and new guidance was released in February (DVLA, 2019a; 2019b). The DVLA now classifies both flash and continuous glucose monitoring as valid methods to prove fitness to drive for non-commercial Class 1 drivers.
The Libre’s manufacturer, Abbott, announced in November 2017 that the device would be made available on the NHS. This was closely followed by statements from various big players in the healthcare and diabetes world, including the Regional Medicines Optimisations Committee (RMOC), Diabetes UK, NHS England and the Association of British Clinical Diabetologists (ABCD). The RMOC started by releasing a statement with the aim of reducing initial variation of prescribing (RMOC, 2017). The ABCD followed suit by setting up a national audit to assess flash monitoring usage. Some Clinical Commissioning Groups (CCGs) argued that evidence from clinical trials was lacking, although a large amount of real-world and lived-experience data were available, and there were concerns about the glucose-reading lag time, accuracy and reliability. Controversy arose as the “battle for the Libre” turned into a postcode lottery across the UK, with some areas refusing to allow access on an NHS prescription.
Each sensor costs £48 to people who are self-funding or £35 to the NHS. This becomes cost-neutral to the NHS when the person with diabetes is using eight or more blood glucose test strips a day. Access to the Libre was debated by the All Party Parliamentary Group for Diabetes last July. Professor Gerry Rayman explained that clinical studies had shown that, in people on multiple daily injections, the number of test strips had being significantly reduced after prescribing the Libre. There were also other significant benefits, including reduced hospital admissions, improved HbA1c and other standard clinical measures, as well as improvements in users’ quality of life.
The current NHS England (2019) criteria for access to the Libre are as follows:
- People with type 1 diabetes, and/or with any form of diabetes on haemodialysis and on insulin treatment, who require glucose testing more than eight times a day.
- Pregnant women with type 1 diabetes, for 12 months, including post-delivery.
- People who cannot routinely self-monitor (e.g. for reasons of disability).
- People whose specialist multidisciplinary team decide would have occupational benefit.
- Those with psychosocial situations that warrant a 6-month trial.
- Previous self-funders with type 1 diabetes, where those with clinical responsibility are satisfied that one or more of these criteria were met prior to self-funding and that the user has shown improvement in HbA1c.
- Recurrent hypoglycaemia in type 1 diabetes.
In addition, NHS England recommends that:
- Education on flash glucose monitoring is provided (online or face to face).
- The person is agreeable to scanning more than 70% of the time.
- Users agree to regular reviews with the clinical team.
- Users have had previous attendance at structured diabetes education.
The Southampton City experience
Southampton City CCG has an estimated population of 14500 people with diabetes (all types). The team has been up and running with FreeStyle Libre education and clinics since February 2018. A great deal of time was spent working together with the CCG to create a cost-effective and efficient pathway for people who were going to be able to access the Libre in accordance with the RMOC (2017) criteria.
We set up monthly clinics based in the hospital diabetes centre, for groups of 12–18 people at a time. This was to ensure the peer-support aspect was also encouraged. We tried to make the groups a mix between people who had been self-funding previously and those who were new to the Libre, hoping that they could all learn from each other. The clinics were staffed by an Abbott representative and two diabetes specialist nurses (DSNs). We have completed a total of 15 clinics to date. We have had 100% attendance at 98% of those clinics.
In Southampton City CCG, we currently have 217 people using the Libre. With an estimated population of 1432 people living with type 1 diabetes, this equates to an uptake rate of 15.2%. NHS England (2019) has planned for the proportion of people using the Libre to reach 20%, so we are still lagging slightly.
Study aim
The aim of gathering this evidence was to acquire real-world data and knowledge about whether the FreeStyle Libre has been helpful for people with type 1 diabetes. We wanted to focus on lifestyle factors and the effect that the Libre had on these.
Method
We received referrals from primary care and our own consultant-led multidisciplinary team clinics from within our service. People were triaged and put into clinics in order of referral, unless there was a more urgent clinical need, such as severe hypoglycaemia, hypoglycaemia unawareness and diabetic ketoacidosis (DKA).
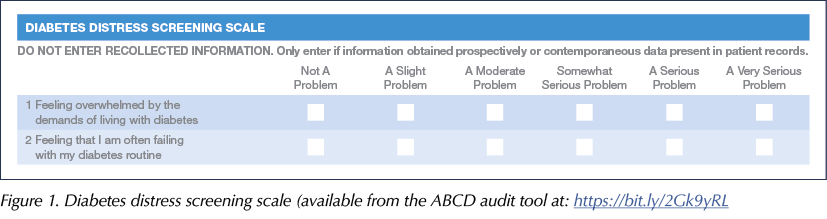
Everyone attending the clinic was asked to fill out the ABCD audit tool forms (available at: https://bit.ly/2Gk9yRL) with a DSN, in order to measure certain aspects of diabetes care before and after use of the Libre. This included the Gold score of hypoglycaemia awareness (Gold et al, 1994), how many blood glucose test strips they were currently using per day, the number of hospital admissions for DKA or hypoglycaemia, HbA1c levels and diabetes distress levels. To get a quantifiable score for diabetes distress, we assigned numbers from 0–5 to the scale shown in Figure 1, with 0 representing the least distress and 5 the most. Adding the scores from the two questions gave a minimum score of 0 and a maximum of 10.
We saw 12–18 patients a month in the starter group clinics. Most were completely new to the Libre, but some had self-funded previously. Those attending were given a 1-hour presentation about the Libre, including aspects such its benefits and limitations, and practical use of the device (e.g. questions such as whether it could be worn in an X-ray machine). All attendees applied a sensor during the clinic, were provided with a set-up reader and were informed about the process of the 6-month trial. The DSN wrote to the GP at this stage asking for further long-term prescriptions for the sensors.
A DSN called the users after 1 month and 6 months to assess their progress and how they felt they were getting on. The DSN also assisted with any help needed to overcome issues with IT or prescribing. At the 6-month follow-up, the DSN repeated the ABCD audit forms with the users to assess their use of the Libre and the changes they had experienced.
Results
We now have 217 people using the Libre.
- Three left the trial before the 1-month review: one moved out of the area and two decided they did not want to continue using the device.
- Six had not engaged with the process at the 6-month review: they were no longer requesting the sensors on prescription or uploading to LibreView. We do not have any further data on these patients as they failed to respond to our communications.
- So far we have had seven groups complete the 6-month review, with a total of 89 users.
- The remainder are still in the trial period.
HbA1c
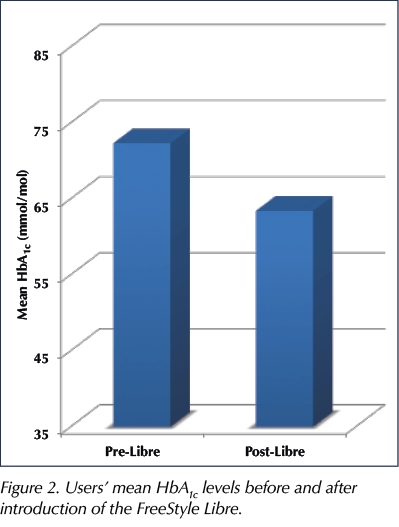
- Of the 89 users who completed the 6-month trial, 75 (84%) had reductions in HbA1c, by varying levels.
– The average drop in HbA1c was 8.9 mmol/mol (range, 3–31 mmol/mol; Figure 2). - Four users increased their HbA1c, but they started with a very low reading, so this actually lessened the frequency of hypoglycaemic episodes they were having. This would still count as a positive change.
Time in range
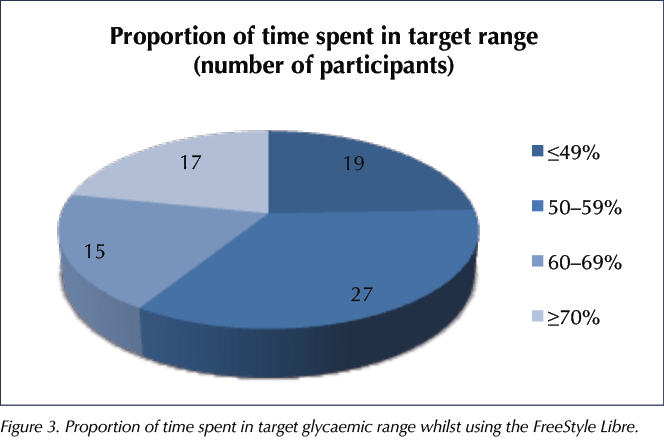
HbA1c does not always give a fully accurate indication of lifestyle factors and glycaemic control. The way best way to determine this would be to look at the amount of time spent in the target glycaemic range (Figure 3). The target was individualised for each user but was typically 4–10 mmol/L.
Vigersky and McMahon (2019) estimated that each 10% increase in time spent in range corresponded to a reduction in HbA1c of 8.5 mmol/mol (0.78%). In our audit, of the 78 users with data available, 32 (41%) spent ≥60% of time in range, roughly equivalent to an HbA1c of 58 mmol/mol (7.5%) or lower.
Hypoglycaemia unawareness
The Gold score was used to measure users’ hypoglycaemia awareness. All 89 users improved or maintained their level of hypoglycaemia awareness. The mean Gold score decreased from 2.66 to 1.25 (Figure 4).
Diabetes distress
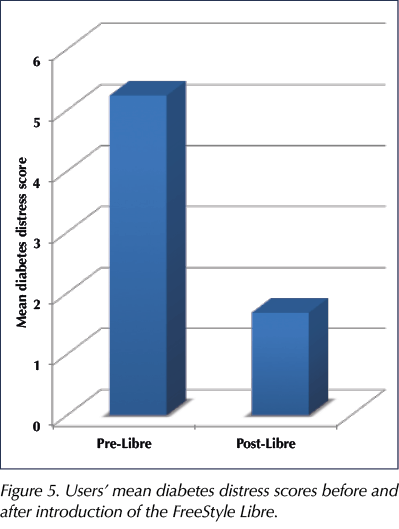
Overall, 85 of 89 users improved their diabetes distress score, with the mean score reducing from 5.24 to 1.68 (Figure 5). We found that the Libre has given a lot of people significant relief from the daily routine of checking their blood glucose, enabling them to gain much greater freedom.
We also received reports of significant reassurance and lowered distress seen by family members and partners of the people using the Libre, although this outcome was not measured. This sometimes related to use of the LibreLink app, which allowed family members also to view blood glucose data.
It is important to note, however, that in a small minority (four of 89 users), the Libre increased anxiety, actually causing some distress, due to the feeling of never being “adequately controlled”. The distress appears to be caused by social media and peer influencers having “perfect data” that users aspire to. Some also reported feeling slightly overwhelmed by the sheer amount of data and were struggling with how to interpret this alone. One user explained how they felt the Libre meant they were reacting to very small changes in their glucose profiles and therefore, perhaps, overtreating when before they would have not noticed them. Another problem that many anticipated would be the changeover from finger-prick testing to reliance on the Libre. Others highlighted that they like the use of bolus-advisor meters, and that the Libre does not currently allow for the two to be interchangeable. The Libre can be used as a blood ketone meter as well, however. This meant that people using it did not have to carry around multiple testing devices, which alleviated some distress.
Blood glucose testing strips
All users reduced the amount of test strips they were using – quite often from very high numbers (going from 10–12 to 0–5 strips per day).
Prior to 14 February 2019, we did advise users to test their blood glucose level with a capillary sample before and during driving. However, it would appear that most users relied solely on the Libre despite this advice. The DVLA legislation has now officially changed, allowing continuous and flash glucose monitoring readings for drivers with a Class 1 licence. This change will likely further reduce the need for blood glucose testing strips on prescription.
It was found that, on three occasions, some users struggled with the change from using bolus advisor meters to using the Libre. We make sure all people going through the Libre education have undertaken a form of structured type 1 diabetes education, and therefore will have some awareness of carbohydrate counting without the smart meter. Currently, Libre data cannot be used to give bolus advice via the reader. Nonetheless, nobody stopped using the Libre in favour of returning to their smart meter. One person, however, continued to use both.
Notably, whilst the data collected in this study show that test strips are not being used as often, our CCG reported no fall in test strip prescribing. We know that, despite users not using as many blood ketone and glucose testing strips, many GPs and pharmacists may still be issuing these out of habit or caution. We left blood glucose testing strips on repeat prescriptions, due to DVLA guidance and for safety reasons (e.g. for events such as sensor failure). Therefore, the local CCG data, which showed no drop in test strip prescribing, might not be representative of the actual need for test strips.
Acute admissions
The cost of a DKA admission is estimated to be around £2064 (Dhatariya et al, 2017). About half of DKA hospitalisations could be avoided with better outpatient and self-delivery of care (Joint British Diabetes Societies for Inpatient Care, 2013).
Of the 89 users who have been through the 6-month review process, the following admissions were recorded in the year prior to Libre uptake:
- Seven had a total of 14 DKA admissions between them.
- Eight had a total of 10 admissions for hypoglycaemic events and other appointments related to their diabetes.
- Five called for a total of 11 ambulances.
Following introduction of the Libre, none of the patients with previous hospital admissions had an admission in the 6-month follow-up.
Insulin pump use
Five of the 89 users no longer meet criteria to have an insulin pump, due to their improvements in HbA1c. An insulin pump is an expensive treatment, costing around £2000 to £3000 for the pump and an additional £1500 per year for consumables (Diabetes UK, 2019). Reducing the need for insulin pumps could result in substantial cost savings to the NHS.
Future projects
Due to the success of introducing the Libre in Southampton, we are now looking at working with a specific set of patients with type 2 diabetes. This will be a small initial project but will work in the same way as with the type 1 diabetes cohort. Those taking part will similarly be offered 6-month use of the Libre. The aim is to look at reducing cost burden for the NHS but, more importantly, improving the life experience for these people. We aim to focus on high-risk patients, such younger people, those who are frail, those on long-term steroid treatment and, perhaps, those who are house-bound. We are also considering the possibility of using the Libre in newly diagnosed people with type 2 diabetes, who might benefit from a 3-month trial in order to learn about their diabetes and blood glucose levels.
Going forward, we are also looking at streamlining our pathways to make them easier to incorporate into our daily workload. This will also work well for people with diabetes who are accessing the Libre via primary care.
Service issues
At each stage of the Libre clinics we have required at least two DSNs, which has created an additional burden of administration work on top of our day-to-day clinical work. We have also found that some of the people with diabetes need IT support to get started with LibreView and the various platforms available to them. This is quite time-consuming, as we often had to contact someone who specialises in IT.
The use of clinic time evolved and we saw that we needed to adapt slightly to use the time more efficiently. During the last six clinics, we have started to set people up on the various smartphone apps and LibreView whilst they are with us. This has meant a reduction in our time used, and also less confusion for the people using the device.
There is also the administration side of things. The referrals needed to be triaged, and admin staff need to book up clinics. There is also the need to stay on top of the ordering of stock from the manufacturer.
Discussion
Since the introduction of the FreeStyle Libre, we are seeing a change in not only how people with diabetes manage their condition but also how healthcare professionals are supporting them. This is evident in the level of engagement for some users and the change in the amount of blood glucose and ketone test strips being ordered. Our findings echo the recent results from the national ABCD audit into the Libre, which were announced at the Diabetes UK Professional Conference (Deshmukh et al, 2019).
Positive findings have been seen in the frequency of acute calls to 999 and out-of-hours services, and/or subsequent admissions with DKA and hypoglycaemia. It has provided a lifeline to some, from the person who was having disabling hypos and ambulance call-outs twice a week, to the mother who is now sleeping better at night. We have seen that people are now self-managing their condition alongside physically demanding jobs and sport. Patients are increasingly accessing peer support and learning from others, as well gaining excellent insight. This fuels the desire to learn and engage with education and healthcare teams as well.
From a professional point of view, the Libre sensor has opened up a multitude of possibilities for diabetes management. We are now reacting to data that we would have never seen before. We can see where rapid-acting insulin is too quick or too slow in some, and where unrecognised hypoglycaemia may be occurring. The data we are seeing is changing how we look after people and the advice we give them.
April 2019 saw the Libre become available across the NHS to prescribe for people who meet the criteria. The Libre is now accessible to primary care professionals, so it is hugely important that diabetes specialists work hard to up-skill our colleagues in the area of flash and continuous glucose monitoring. It is essential that people with diabetes are supported to manage their diabetes effectively, in order to reduce the risk of long-term complications. A 4 mmol/mol (0.4%) reduction in mean HbA1c would save the NHS an estimated £995 million over the next 25 years (Baxter et al, 2016); thus, even the modest improvements in glycaemic control seen in this audit could generate huge reductions in the cost of diabetes complications.







Key scientific developments presented at the conference.
6 Aug 2025