Glycated haemoglobin (HbA1c) is the agreed international standard for assessing overall glycaemic status in people with diabetes. The timely use of HbA1c is critical to ensuring the best clinical outcome and minimise complications (Holt et al, 2021; Davies et al, 2022). National and international guidance recommend that it is essential that HbA1c should be measured every 3–6 months until is stable on unchanging therapy, and every 6 months thereafter (NICE, 2022a; 2022b; American Diabetes Association, 2024). Despite these recommendations, conformity to this guidance is highly variable, with over half of tests being performed outside the recommended intervals (Driskell et al, 2012).
Highlighting the importance of consistent monitoring, we previously reported that both HbA1c testing frequency and pattern are linked to diabetes control, including the likelihood of achieving target HbA1c levels (Driskell et al, 2014; Duff et al, 2018; Fryer et al, 2022). These findings highlight the importance of implementing approaches to improve monitoring, particularly in those with high HbA1c levels and/or significantly overdue monitoring.
Non-medical healthcare professionals, including pharmacists, physiotherapists, dietitians and podiatrists, are increasingly supporting nurses and doctors in providing some services for people with diabetes (Khan, 2024). The expertise of clinical laboratory specialists, such as biomedical scientists and clinical biochemists, has been less well utilised in this regard.
This study explored the potential of the biochemistry laboratory to ensure that patient-facing healthcare professionals have the best data available to them in relation to the timeliness of testing in those individuals with above target HbA1c.
What we did
Using a monthly, easily understood report sent from the clinical biochemistry laboratory to participating general practices, we sought to provide rapid access to a list of patients with the most sub-optimal control who were overdue their routine HbA1c test. This aimed to assist practices in making more effective use of resources to tackle the backlog of overdue tests by prioritising those who were most at risk.
Anonymised patient-level HbA1c test data, date of test, result and requesting location were extracted from the Laboratory Information and Management Systems at Cambridge University Hospitals NHS Foundation Trust. These data were collected initially for a complete 3-year period from 1 June 2017 to 31 October 2020, and then updated monthly on a 36-month rolling basis until the effectiveness of the programme was analysed in October 2023.
Comparisons were made between two groups of general practices: an “intervention” group, comprising 60 practices who received monthly reports as described below as part of the service development programme, and a “non-intervention” group, comprising 51 practices that did not receive the reports (standard practice).
For each patient, the time intervals between tests and change in HbA1c levels between the most recent tests were calculated. Tests were defined as “overdue” based on previous test result and intervals between tests. Recommended testing intervals were derived from a combination of NICE guidelines (NICE, 2022a; 2022b) and previous studies examining testing interval versus change in HbA1c (Driskell et al, 2012; 2014; Duff et al, 2018; Fryer et al, 2022, Khan, 2024; Holland et al, 2025).
What we found
At baseline (2020), the two groups had a similar proportion of people in the ≥58 mmol/mol (7.5%) category who were overdue an HbA1c test. For those with an HbA1c of >75 mmol/mol (9.0%), there was a larger proportion overdue a test in the intervention group than the non-intervention group (74.3% vs 66.2%; P<0.001).
How the intervention influenced the proportion of people overdue a test
By October 2023, the overall proportion of people overdue a test was 8.9% lower in the intervention group compared to the non-intervention group. This change from 2020 was particularly striking in the >75 mmol/mol category. In the intervention group, the proportion of people overdue a test shifted from being 8.2% higher to 7.2% lower than in the non-intervention group, which corresponded to a 7.8-fold larger reduction in the number of people overdue a test.
How the intervention influenced the days overdue a test
In the 58–75 mmol/mol category, the median days overdue for the intervention group was 14.4% lower than at baseline. For the >75 mmol/mol group, the intervention group showed a numerical reduction in the median days overdue despite starting from a lower baseline, though this just failed to reach the statistical significance threshold (P=0.059).
How the intervention influenced HbA1c
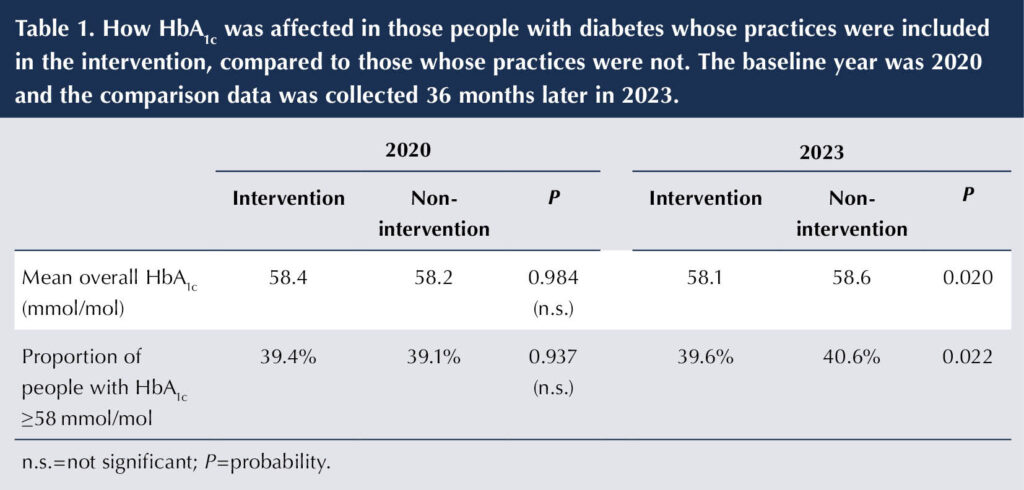
The intervention group also showed a fall in median HbA1c level, while the non-intervention group showed deterioration in both HbA1c and in the proportion of HbA1c ≥58 mmol/mol (Table 1), resulting in statistically significant differences in both parameters (P=0.020 and P=0.022, respectively, for comparison between the intervention and non-intervention groups at 3-year follow-up in 2023). All remained significant after adjustment for practice characteristics (age, sex, social deprivation, list size and diabetes prevalence) and pre-intervention levels.
Relevance
Our findings indicate that clinical laboratories can support nurses in primary care, community clinics or secondary care teams, and doctors, by facilitating targeted monitoring of those without well-controlled diabetes. Provision of succinct monthly reports identifying patients overdue HbA1c testing can reduce the number of overdue tests. We showed that this can lead to improved glycaemic control and a higher proportion of individuals achieving target levels. As far as we are aware, this is the first study to demonstrate the effectiveness of such a laboratory-led intervention.
While small at the individual level, extrapolating the 1% net reduction in the proportion of those with HbA1c ≥58 mmol/mol due to the intervention would mean that approximately 18000 additional people in the UK (4.4 million people with diabetes) could be moved from the ≥58 mmol/mol to the <58 mmol/mol category with a straightforward laboratory-led intervention.
Overall, there was a reduction in the proportion of tests and days overdue between 2020 and 2023, as the early part of this period corresponded with the UK “lockdown” periods of the COVID-19 pandemic when many fewer tests were done (Holland et al, 2023), after which there was a steady increase in the rate of HbA1c testing across the UK and elsewhere. This phenomenon affected both the intervention and non-intervention arms equally.
For those with HbA1c of >75 mmol/mol, the intervention group had a higher proportion overdue a test at baseline (74.3% vs 66.2% in the non-intervention group; P=0.001). However, this was reversed following the implementation of the reports by 2023, with the intervention group having a significantly lower proportion overdue a test (56.4% vs 63.6% in the non-intervention group; P=0.001). This is supported by the longitudinal data and suggests that the intervention precipitated a targeting of both 58–75 and >75 mmol/mol groups.
The intervention not only improved monitoring frequencies in this group, but also resulted in reduction of HbA1c levels. This is in keeping with previous studies showing that improved monitoring is associated with better diabetes control (Driskell et al, 2012; Anjana et al, 2015; Fryer et al, 2022; Khan, 2024; Holland et al, 2025). The reasons why regular and timely HbA1c testing is associated with better glycaemic control may relate to primary care healthcare professionals being alerted to medication review if HbA1c is regularly checked and to increasing the awareness of the patient about their own diabetes control. This is relevant because keeping to a regular testing schedule does enhance a sense of self-efficacy (Orr et al, 2006).
Conclusion
Our findings indicate that clinical laboratories can support targeting monitoring in high-risk patients. Provision of succinct monthly reports identifying patients overdue testing to the clinical team can reduce the number of overdue tests, and hence improve diabetes control and achievement of target levels of HbA1c.
In the future, clinical laboratories could support nurses and doctors involved in diabetes care delivery by contacting overdue patients directly to arrange testing. We have shown that this does improve HbA1c for a considerable number of people who are above the target range, which has implications for future health trajectory and reduced risk of complications developing.
Compliance with ethical guidelines
This study is part of a service development initiative to increase the quality of laboratory test requesting. Hence, it includes a service evaluation and audit of local practice over time with a view to implementing a service development intervention to enhance the clinical laboratory service and improve conformity to recommendations on monitoring intervals. Accordingly, this study was not considered to be research using the decision tool provided by the UK Health Research Authority and did not require NHS Research Ethics Committee review. All data extracted from Laboratory Information and Management Systems were anonymised.





Developments that will impact your practice.
29 Aug 2025