Since 2009, new therapies have joined the treatment algorithm for the management of hyperglycaemia in type 2 diabetes. These include glucagon-like peptide-1 receptor agonists (GLP-1 RAs), dipeptidyl peptidase-4 (DPP-4) inhibitors and sodium–glucose cotransporter 2 (SGLT2) inhibitors. The American Diabetes Association and European Association for the study of Diabetes consensus guideline recommends that, in people with type 2 diabetes and established atherosclerotic cardiovascular disease, treatment for hyperglycaemia should be diet, lifestyle change and metformin, followed by, if this does not achieve target HbA1c levels, an agent proven to reduce cardiovascular mortality risk (Davies, 2018). To date, the latter includes the GLP-1 RAs liraglutide and semaglutide, and the SGLT2 inhibitors empagliflozin and canagliflozin.
GLP-1 RAs
There are currently four GLP-1 RAs available in Ireland: exenatide, liraglutide, dulaglutide and the recently launched semaglutide. All diabetes medications are covered under the Long Term Illness Scheme with the Health Service Executive (HSE) Ireland. At present, the HSE reimburses liraglutide up to the dose of 1.8 mg.
GLP-1 RAs assist glucose homeostasis through several mechanisms (Figure 1). Satiety is primarily achieved via a delay in gastric emptying and absorption of carbohydrate from the gastrointestinal tract. The agents promote insulin secretion by the pancreatic islet cells and inhibit gluconeogenesis by the liver. There is also the added benefit of not inducing hypoglycaemia.
GLP-1 RAs are contraindicated in people with a history of pancreatitis or thyroid medullary carcinoma. They can be used in patients with an estimated glomerular filtration rate over 15 mL/min/1.73 m2.
This article focuses on the GLP-1 RA liraglutide and presents an Irish audit highlighting its use in people with type 1 diabetes.
Studies of GLP-1 RAs in type 2 diabetes
GLP-1 RAs have shown effects on glycaemic control and weight loss in clinical trials. A Cochrane systematic review of GLP-1 RAs in people with type 2 diabetes revealed HbA1c reductions of approximately 11 mmol/mol (1.0%) and weight reductions of 1.5–2.5 kg compared with baseline (Shyangdan et al, 2011). This weight loss was further confirmed in another meta-analysis of 21 trials (Vilsbøll et al, 2012).
Two GLP-1 RAs have shown cardiovascular benefits in cardiovascular outcome trials. Compared with placebo, liraglutide significantly reduced the risk of major adverse cardiovascular events (MACE) by 13%, including a 22% risk reduction in cardiovascular death, a 12% reduction in non-fatal myocardial infarction (MI) and an 11% reduction in non-fatal stroke (Marso et al, 2016a). There was also benefit in terms of renal outcomes. Similarly, semaglutide resulted in a 26% reduction in MACE risk compared with placebo, including a 26% reduction in non-fatal MI and a 39% reduction in non-fatal stroke (Marso et al, 2016b).
Despite similar mechanisms of action, the agents within the GLP-1 RA class differ in their clinical effects due to differences in structures and pharmacokinetic profiles (Pratley et al, 2018). Liraglutide has resulted in greater HbA1c and weight reductions in comparison with the other GLP-1 RAs in three clinical trials (Buse et al, 2009; Buse et al, 2013; Dungan et al, 2014). Liraglutide has also shown superiority to other classes of oral agents – DPP-4 inhibitors and SGLT2 inhibitors – in some studies (Pratley et al, 2010; Pratley et al, 2011; Lorenzi et al, 2017).
Efficacy and safety of liraglutide in type 1 diabetes
Incretin-based therapies (GLP-1 RAs and DPP-4 inhibitors) have the potential to protect beta-cell mass, trigger insulin release and suppress glucagon release (Nauck et al, 2009). Two key studies have examined the efficacy and safety of liraglutide in type 1 diabetes. ADJUNCT ONE was a 52-week, double-blind, treat-to-target study conducted in 1398 people randomised 3:1 to receive liraglutide (1.8 mg, 1.2 mg or 0.6 mg) or placebo, in 177 centres in 17 countries (Mathieu et al, 2016). The results showed weight loss of 4.9 kg, 3.6 kg and 2.2 kg, respectively, compared with placebo, and small improvements in HbA1c. Insulin total daily dose (TDD) was also reduced by 5% and 2% with liraglutide 1.8 mg and 1.2 mg; however, it increased by 4% with the 0.6 mg dose. The main complications were increased risk of symptomatic hypoglycaemia with all doses, and hyperglycaemia with ketosis for the 1.8 mg dose only.
ADJUNCT TWO was a 26-week, double-blind, parallel-group trial with 835 people randomised 3:1 to liraglutide or placebo (Ahrén et al, 2016). Outcomes revealed significant reductions in HbA1c, weight and TDD, and improved quality of life. Again, the complications were hypoglycaemia and, with the 1.8 mg dose, ketosis. The authors stated that these side effects limited the clinical use of GLP-1 RAs in people with type 1 diabetes.
Dublin audit
While they are not currently approved by the US Food and Drug Administration for this indication, St Vincent’s Healthcare Group in Dublin uses GLP-1 RAs off label as adjunct therapies in select patients with type 1 diabetes to stabilise glucose control, reduce weight and reduce insulin TDD. The aim of this retrospective audit was to establish real-world effects of liraglutide use in people with type 1 diabetes.
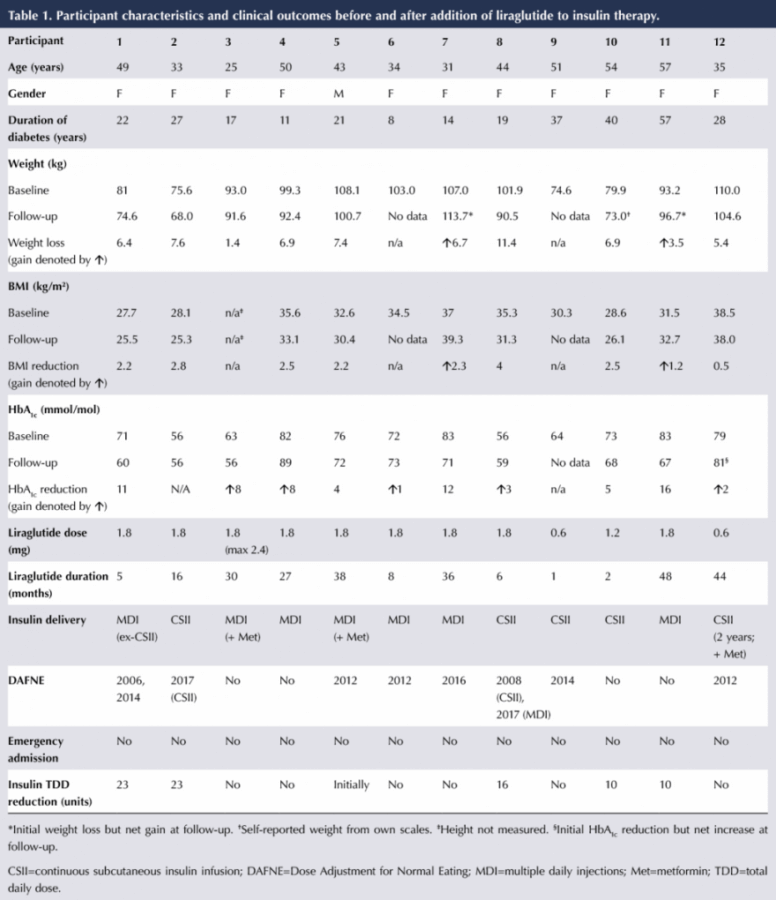
There are currently 8783 active patients listed on our dedicated diabetes database between the three hospitals in St Vincent’s Healthcare Group: 5677 in St Vincent’s University Hospital, 1692 in St Michael’s Hospital and 1414 in St Vincent’s Private Hospital. The audit commenced in March 2018 and finished in April 2018. The database revealed 554 people on liraglutide. Those with type 2 diabetes and two brothers with monogenic diabetes were excluded. Twelve people with type 1 diabetes were identified and evaluated.
Liraglutide was initiated at the dose of 0.6 mg once daily and titrated up to 1.2 mg or 1.8 mg according to tolerance. One patient moved to a 2.4 mg dose temporarily. Liraglutide is licensed up to 3.0 mg to treat obesity; however, as stated previously, the HSE in Ireland only reimburses up to 1.8 mg in people with diabetes.
Results
The individual characteristics and clinical outcomes of the 12 participants are shown in Table 1. Over a mean treatment duration of 22 months (range, 1–48 months), eight participants lost weight, with an average loss of 6.7 kg. Two people increased their weight (by 6.7 kg and 3.5 kg, respectively) and two had no weight data at follow-up as they had not been back for clinic review. Mean BMI in those who lost weight was reduced from 32.7 to 29.9 kg/m².
HbA1c fell in five people, by a mean of 9.6 mmol/mol (0.9%); however, it rose by a mean of 4.4 mmol/mol (0.4%) in five and stayed the same in one. One participant had not had an HbA1c measurement at follow-up. Insulin TDD was reduced in five participants, by an average of 16.4 units.
There were no episodes of hypoglycaemia (defined as a blood glucose of <4 mmol/L) or diabetic ketoacidosis whilst on liraglutide therapy, and no emergency hospital admissions. Patients 6 and 7 have discontinued treatment as they are planning pregnancy.
Discussion
In our practice, GLP-1 RA initiation is considered as a potential adjunct therapy in overweight and obese people with type 1 diabetes. Clinical review of patients can determine whether GLP-1 RAs may be beneficial to assist weight loss in this cohort, even if the practice is off-label. The current audit suggests that, in certain patients with type 1 diabetes, the GLP-1 RA liraglutide had modest benefits in terms of glycaemic control, weight loss and insulin dose reduction. However, a subset of patients did not respond to this therapy.
Liraglutide was well tolerated, with no episodes of hypoglycaemia or diabetic ketoacidosis. Patients were made aware of the increased risk of hypoglycaemia that occurs when GLP-1 RAs are added to insulin therapy, due to effects on gluconeogenesis and glycogen stores in the liver, and were advised to be vigilant. Insulin dose reduction was recommended, and five participants reduced their TDD by a mean of 16.4 units. A reduction in insulin TDD can assist with weight loss in patients who are overweight.
A large proportion of this cohort had completed the Dose Adjustment for Normal Eating (DAFNE) structured education programme, which has the benefit of allowing patients to find their own insulin TDD requirement. A lot of patients on inappropriate insulin doses increase their food intake to avoid hypoglycaemia, and this can lead to weight gain. The DAFNE programme assists them to identify their own individual dose requirements, and thus a proportion of DAFNE participants lose weight following the programme. In addition, while DAFNE allows flexibility in the foods eaten, healthy eating is advocated in all participants who are not within a normal BMI.
Eight of the current cohort had completed the DAFNE programme: six had completed regular DAFNE (one had completed it twice), two had completed the insulin pump course and one had completed both regular and pump courses. DAFNE graduates did not appear to be more likely to benefit from liraglutide than non-graduates.
The sample cohort in this audit was small, which is appropriate as we are not recommending widespread initiation of GLP-1 RAs in patients with type 1 diabetes. In our practice, adjunct therapies are only maintained if they have the desired impact in terms of weight loss or reductions in HbA1c. Due to the costly nature of GLP-1 RA therapy, the decision is made to discontinue treatment in non-responders.
Conclusion
Discretionary use of GLP-1 RAs in select cases may confer benefits in people with type 1 diabetes who are overweight. Weight gain in people with type 1 diabetes can be attributed in part to inappropriate insulin doses, as we have seen significant weight loss in graduates of the DAFNE structured education programme in post-course follow-up.





Study provides new clues to why this condition is more aggressive in young children.
14 Nov 2025