The prevalence of diabetic foot ulceration is increasing and its management poses a serious challenge to healthcare professionals. It is estimated that the prevalence of diabetic foot ulcers in England is about 58,000 (Kerr et al, 2019). Neuropathic ulcers are the point of entry of pathogens, leading to osteomyelitis. This has become the leading cause of non-traumatic major limb amputation in the UK, with about 7,000 patients losing a limb every year (Kerr et al, 2019). Although specific data regarding amputations due to diabetic foot ulcers are sparse in the literature, some authors quote amputation rates of up to 24% following non-healing ulcers (Pemayun et al, 2015). Amputations in people with diabetes have high morbidity and mortality. The annual cost of management of these is estimated to be over £1 billion (Kerr et al, 2019).
Multidrug resistance, poor bone penetration of antibiotics and coexisting vasculopathy make management of diabetic foot infection a major challenge (Aragón-Sánchez 2010; 2011). The use of a local antibiotic is an attractive option in these patients.
We review our results of limb salvage surgery for diabetic foot infection since the introduction of a new local antibiotic carrier, and present a short review of mechanics of local antibiotic carriers.
Methods
This paper presents the combined experience of two acute hospital trusts in the UK in the use of an antibiotic-loaded calcium sulphate hydroxy apatite biocomposite for the management of diabetic foot osteomyelitis. (CERAMENT G®, BONESUPPORT, Lund, Sweden).
Patient demographics, co-morbidities, presenting features and pre-operative investigations were reviewed. Foot ulcers were classified according to the University of Texas staging system (Table 1; Monteiro-Soares et al, 2020).
Inclusion criteria
This review included all patients who underwent operative management of diabetic foot disease, had CERAMENT G, as adjuvant to debridement or reconstructive surgery and managed under the supervision of the multidisciplinary foot team at United Lincolnshire Hospitals NHS Trust and East and North Hertfordshire Hospitals NHS Trust.
Patients were assessed and managed by our multidisciplinary diabetic foot teams, which include a diabetes physician, an orthopaedic foot surgeon, a vascular surgeon, a podiatrist, a microbiologist and a musculoskeletal radiologist.
Both units have established protocols for the management of diabetic foot disease. This includes a detailed clinical assessment from an orthopaedic, vascular and endocrine point of view. The patient is given a full blood workup, including HbA1c, renal function and inflammatory markers. Weight-bearing antero-posterior and lateral radiographs are taken. The criteria for a diagnosis of osteomyelitis were: ulcer over a bony prominence, presence of discharging sinus, visible exposed bone, positive probe to bone test or red swollen toe with ulcer (Lipsky et al, 2015).
Patients without palpable pulse or only a monophasic pulse on a hand-held Doppler were given a duplex scan. If the distal run-off was poor, an angiogram was organised. Foot perfusion is improved, where applicable, with angioplasty. In cases of minor forefoot procedure where the risk of a vascular procedure is high, we undertake an orthopaedic procedure and resort to a vascular procedure only in case of non-responsiveness of the wound to debridement and antibiotic.
In this study, we included 48 feet in 47 patients who were operated on between February 2017 and November 2019. Our exclusion criteria were gentamicin allergy and significant renal disease.
We have adopted a standard protocol for surgical management, which involves a single stage procedure (Aragón-Sánchez, 2010). Patients were operated on under regional or general anaesthesia and application of tourniquet when possible. Sinus tracts were excised. Although adequate infected bone and soft tissue were debrided to prevent recurrence, we were careful to retain enough bone and soft tissue to maintain foot function and stability. Multiple deep bone and soft tissue samples were obtained for microbiology analysis.
The level of bone and soft tissue resection was determined by bone quality and extent of infection, as assessed on MRI scans and intra-operative findings combined with surgeon experience. Bone resection was carried out to de-tension soft tissues and enable healing. This included excision arthroplasty of the metatarsophalangeal joints in forefoot ulcers and partial calcanectomy in heel ulcers (Table 2)
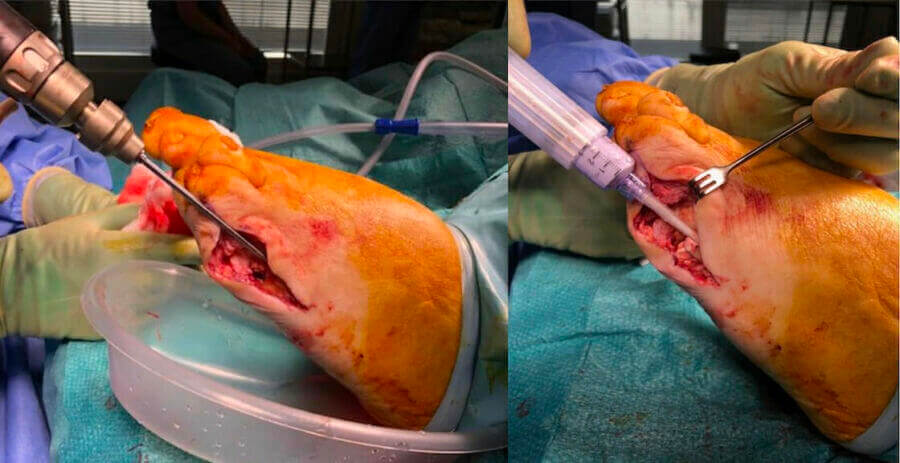
The next stage involved delivery of the local antibiotic into the bone. CERAMENT G (was delivered into bone using the Silo technique for midfoot and hindfoot and intramedullary retrograde filling for forefoot (Figures 1 and 2; Drampalos et al, 2019). The mean volume of biocomposite used was 5 ml, which contains 87.5 mg (175 mg/10 ml) of gentamicin. When possible, the wound was closed primarily without soft tissue tension or managed with dressings and vacuum-assisted closure. Patients were offloaded in a plaster or walking boot until complete wound healing.
Culture-specific antibiotics were administered as per advice from microbiologists. Post-operative management was under the care of the multidisciplinary foot team as per national guidelines (National Institute for Health and Care Excellence, 2019).
Results
The patients’ average age was 55 years (range 34–83 years). The majority had forefoot infections, with 32 having osteomyelitis of the metatarsals, while eight had hindfoot infections and eight had midfoot infections. All patients had other comorbidities related to diabetes; and 17 were ASA type 2 and 29 were ASA type 3. The foot ulcers were classified as University of Texas grade 3B (40 ulcers) or 3D (eight ulcers).
Six patients had angioplasty prior to orthopaedic intervention. In addition to debridement and sequestrectomy, 22 patients had other reconstructive procedures (Table 2).
A total of 28 feet had secondary healing with or without negative pressure wound therapy, 17 were closed primarily and three had skin grafts (Table 3).
At a mean follow-up of 33 months (range 13–49 months) we achieved infection control and healing in 42 feet (88%), and 39 patients (83%) were mobilising and fully weight bearing with custom-made surgical shoes.
Three patients required below-knee amputation; thus we had a 94% limb salvage rate. All three of these patients had heel ulcers and calcaneal osteomyelitis. Two of these had significant adherence issues and one patient had significant microvascular disease.
Three patients had non-healing and persisting ulcers at the time of most recent follow-up. All three of these patients were unable to comply with offloading due to social issues.
The average time to wound healing was 16 weeks (range 3–24 weeks), as shown in Figure 3. C-reactive protein had a pre-operative mean of 53.7 (range 1–152) and reduced to 10.8 at 4 weeks post-operative (range 1–25).
We isolated a variety of organisms, including Pseudomonas, Staphylococcus, mixed Gram-negative flora, Streptococcus and trichophyton in 36 out of 48 intra-operative cultures (Table 4). The patient who had the trichophyton fungal growth responded well to a course of antifungal therapy. The remainder of the cultures were negative for any organism; this was possibly due to the patients having IV antibiotics before coming into our care. Two patients had repeat debridement and one had a recurrence of infection after complete healing.
There were no other recurrences or systemic side effects during the study duration. We observed milky white discharge from the biocomposite which settled down after the first post-operative week and did not cause any further concerns.
Discussion
Osteomyelitis in the diabetic foot can be difficult to manage due to vasculopathy, neuropathy and systemic disease. Proper surgical debridement is key because impaired immunity and presence of biofilm can lead to infection persisting (Stewart, 2002). Biofilm is composed of a matrix of polysaccharide, protein, and other molecules with colonies of bacteria. These reduce the efficacy of antibiotics due to the slow replication of the organism and slow metabolism (Donlan and Costerton, 2002; Patel, 2005).
It is known that systemic antibiotics do not achieve adequate levels in bone defects (Ferguson et al, 2017). In such cases, local antibiotic delivery systems are particularly valuable because they can diffuse into areas with poor perfusion and achieve very high local concentrations without systemic toxicity (Cierny et al, 2006).
Carriers for local antibiotics may be categorised as non-biodegradable polymers, bone grafts, bone graft substitutes, collagen, gels and aqueous solutions (Stravinskas et al, 2016). Antibiotic release from a non-biodegradable polymer such as polymethylmethacrylate (PMMA) is dictated by surface area and concentration gradient (Ferguson et al, 2017). These polymers have been shown to create very high levels for the first 2–3 days and then elute at levels below the antibiotic’s minimum inhibitory concentrations for a very long time, leading to antibiotic resistance (Patel, 2005; Ferguson et al, 2017). PMMA can also be a source of infection recurrence due to formation of biofilm on its surface and require a further procedure for removal. Biodegradable carriers, collagens or gels can release local antibiotic at very high and uncontrolled rates.
Stravinskas et al (2016) studied the antibiotic-eluting properties of CERAMENT G in vitro and in vivo. In vitro studies with Ringer’s lactate showed high initial peak in the gentamicin concentrate followed by a maintained level above 4 mg/ml for 28 days. The high level of bactericidal antibiotic from CERAMENT G acts at an important time when most residual organisms will be in the planktonic form. This agent delivers a burst of gentamicin with high local concentration and maintains levels that are 100 times above the minimum inhibitory concentrations for Staphylococcus aureus and Pseudomonas (McNally et al, 2016).
This is similar to in vivo studies that were carried out in patients with cortico-medullary osteomyelitis, hip revisions and hip fractures (BONESUPPORT, data on file; Stravinskas et al, 2016). No patient demonstrated serum levels that would be associated with any systemic toxicity. They have demonstrated an elution pattern that starts with a burst, which decreases with time and is maintained above minimum inhibitory concentration level for 4 weeks.
CERAMENT G was developed by adding antibiotics to a biphasic ceramic bone graft substitute and is marketed as a synthetic injectable composite. The injectable form means that this agent is able to coat the bone and fill small and large defects. The biphasic resorption of calcium sulphate and hydroxyapatite forms an osteoconductive scaffold and leads to an early biological response (Cierny and DiPasquale, 2006; Stravinskas et al, 2016; Niazi et al, 2019). It has been shown that CERAMENT G injected into osteomyelitis bone voids decreased the rate of infection and increased bone formation in a rat osteomyelitis model (Dvorzhinskiy et al, 2016).
McNally et al (2016) have reported on a series of 100 patients with chronic osteomyelitis that they managed with single stage CERAMENT and have reported 96% successful results.
Studies showing local antibiotic carriers in diabetic foot infections are sparse, with a recent two-centre series being the only one with a good number of patients.
Niazi at al (2019) reported on a review of 70 patients where they used CERAMENT G in diabetic foot infections. They achieved 93% limb salvage and 90% infection eradication, which is similar to our series.
Drampalos et al (2018) reported on a small series of diabetic calcaneal osteomyelitis in which CERAMENT G was used and infection clearance was achieved in all patients. Armstrong et al (2001)demonstrated that calcium sulphate with antibiotics was effective in forefoot diabetic foot infections.
Our series shows that the use of a local antibiotic carrier along with a surgical debridement can achieve infection clearance close to 90% and limb salvage rates close to 95%. Our results are comparable to Niazi et al (2019) in a similar series of patients. We had similar results to their series regarding hindfoot disease, as all our amputations were for hindfoot disease. Moreover, this is a safe procedure with no local or systemic adverse effects. Although these are early results in a small series of patients, longer term outcomes in larger series are required to lay down a firm evidence base to guide treatment.