This issue’s news comes from the 79th Scientific Sessions of the American Diabetes Association (ADA). Tinted boxes summarise the key messages with more detail in the background text. Several presentations had accompanying research papers simultaneously published in peer-reviewed journals. Links to the online published papers are provided.
REWIND (Researching Cardiovascular Events with a Weekly Incretin in Diabetes)
Key points
Dulaglutide once-weekly reduced major adverse cardiovascular events (MACE) by 12% compared to placebo in people 50 years and older with type 2 diabetes and either a previous myocardial infarction (MI) or stroke (20.8%), established cardiovascular disease (CVD; 31.5%) or CV risk factors (just under 70%). This was largely driven by a significant reduction in non-fatal stroke and was similar to MACE reduction with liraglutide in the LEADER cardiovascular outcomes trial (CVOT).
3-point MACE significantly decreased in both those with previous CV events and those with only CV risk factors, with no evidence of heterogeneity between the groups.
The renal component of the composite microvascular secondary endpoint was also significantly reduced (relative risk reduction [RRR], 15%), mainly due to a reduction in the incidence of new macroalbuminuria (RRR, 33%), but this should be considered an exploratory analysis and further studies are ongoing to confirm this.
Cardiovascular results (Gernstein et al, 2019a)
- Primary outcome – first occurrence of the composite endpoint of 3-point MACE (non-fatal MI, non-fatal stroke, CV death including death from unknown causes).
- 3-point MACE occurred in 594 (12%) of the dulaglutide-treated group and in 663 (13.4%) of the placebo group (hazard ratio [HR], 0.88 [95% confidence interval (CI), 0.79–0.99; P=0.026]), representing a 12% RRR.
- MACE reduction driven by a significant non-fatal stroke reduction of 24%.
- No significant difference in:
- CV death
- non-fatal MI
- all-cause mortality.
There was no difference in efficacy between the primary prevention cohort and those with established CVD.
- MACE absolute risk reduction (ARR) was higher and number needed to treat (NNT) lower in participants with established CVD compared to those without.
- NNT to prevent one CV event over 5.4 years with dulaglutide was 18 in those with a previous CV event and 60 in those with CV risk factors only.
Compared to cardiovascular outcome trials (CVOTs) of other GLP-1 RAs, individuals in the REWIND trial were at lower risk of CV events. Two thirds of participants did not have CV disease at baseline. Reflecting on the results, the authors concluded that “dulaglutide could be considered for the management of glycaemic control in middle-aged and older people with type 2 diabetes with either previous CVD or CV risk factors”.
Renal results – exploratory analysis (Gernstein et al, 2019b)
- The renal component of the composite microvascular outcome – first occurrence of:
- new macroalbuminuria (UACR >33.9 mg/mmol), or
- sustained decline in eGFR of ≥30% from baseline, or
- chronic renal replacement therapy.
- 15% significant reduction in the renal component of the composite microvascular endpoint in those treated with dulaglutide compared to placebo, driven by a 23% reduction in new macroalbuminuria.
- No significant difference in sustained decline in eGFR or need for renal replacement therapy.
Owing to non-significant results higher up the statistical hierarchy of the study, the renal data should be viewed as an exploratory analysis or “hypothesis-generating” only.
It is important to remember that SGLT2is and GLP-1 RAs are only currently licensed for glucose-lowering and not for heart failure, renal disease or ASCVD prevention or management. Only initiate these drugs if you know how to use them safely. Further guidance is available in How to use SGLT2 inhibitors safely and How to use GLP-1 receptor agonist therapy safely and effectively.
About the study
Methodology and design
- A randomised, double-blind, placebo-controlled trial involving 9901 participants at 371 sites in 24 countries:
- 46% women
- mean age 66 years
- median duration of diabetes 10.5 years
- median baseline HbA1c 7.2% (55 mmol/mol).
- Participants were randomly assigned to dulaglutide 1.5 mg once-weekly or placebo in addition to other glycaemic therapy and followed for median 5.4 years, aiming to achieve glycaemic equipoise.
- The Lancet simultaneously published the CV and renal results online at http://bit.ly/2GC98af and http://bit.ly/2YxqpLR, respectively.
Limitations
- Nearly half of dulaglutide-treated participants suffered a gastrointestinal adverse event during follow-up, significantly more than the one third in the placebo group. This was similar to those treated with GLP-1 RAs in other CVOTs.
- Around 25% of participants were not on the study drug at the end of the study. This is similar to other GLP-1 RA CVOTs.
- Renal outcomes were exploratory and need to be studied further.
DECLARE-TIMI 58 (Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58
Key points
Initial results of the DECLARE-TIMI 58 CVOT with dapagliflozin were discussed in the Journal previously and were presented at ADA, along with CV data published in Circulation earlier in 2019 and new cardiorenal secondary endpoint data. When reviewing results from this study, it is important to highlight that only 41% had previous CVD and only 7% had an eGFR <60 mL/min/1.73 m2. This is, therefore, a much lower risk population for CV and renal events than in other CVOTs with SGLT2 inhibitors.
The composite cardiorenal secondary endpoint included:
- sustained reduction in eGFR by at least 40% to <60 mL/min/1.73 m2
- end-stage renal disease (ESRD)
- CV or renal death.
Comparing dapagliflozin- and placebo-treated groups, significant reductions demonstrated:
- 24% RRR composite cardiorenal secondary outcome
- 47% RRR composite renal secondary outcome (as above but without CV death)
- 59% RRR ESRD or renal death.
Cardiorenal- and renal-specific endpoints were reduced across a range of pre-specified baseline characteristics, including those with and without ASCVD. These renal data were simultaneously published in The Lancet Diabetes & Endocrinology (Mosenzon et al, 2019).
The speakers concluded that dapagliflozin may be effective in the prevention and treatment of renal complications in a broad range of populations, including those with and without ASCVD, but it should be remembered that SGLT2 inhibitors are not currently licensed for initiation in those with eGFR <60 mL/min/1.73 m2 and must be stopped if eGFR is sustained at <45 mL/min/1.73 m2.
Since only one of the co-primary endpoints was significantly reduced, all other endpoints, including the cardiorenal data presented, must be considered as hypothesis-generating even if statistically significant.
Cardiovascular data
There were two co-primary endpoints:
- 3-point MACE was not significantly reduced in the whole study population.
- Hospitalisation for heart failure (HHF)/CV death demonstrated a significant 17% reduction, driven by a reduction in HHF.
Sub-analyses by baseline MI, CVD and HF
MI was identified as a baseline characteristic of interest to permit sub-analysis. There was a significant 16% RRR in MACE in those with prior MI at baseline, but not in those without previous MI (including those with established ASCVD but no MI; Furtado et al, 2019).
When data from the 59% of people in the study with multiple risk factors but no established ASCVD were examined, the RRR in CVD death/HHF was significant and similar to that recorded in those with established ASCVD but, again, there was no reduction in 3-point MACE in this group. These findings may influence licence change in future.
When data from those with HF at baseline were examined, there was an RRR in HHF seen in those with and without HFrEF, but CV death was reduced only in patients with HFrEF (Kato et al, 2019). There was a tendency for those with worse ejection fraction to receive the most benefit when treated with dapagliflozin.
About the study
DECLARE-TIMI 58 was the largest of the SGLT2i CVOTs to date, with more than 17,000 participants (only 41% of whom had established CVD at baseline) followed for a median treatment time of 4.2 years. 3000 had previous MI at baseline. It is the only SGLT2i CVOT to date to collect baseline HF data and baseline left ventricular ejection fraction in 5000 participants.
- HFrEF (EF <45%) present in 3.9%
- HF but not known to have reduced EF present in 7.7%
- No known HF in 88.4%.
CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation)
Key points
CREDENCE is a CVOT with canagliflozin in those at high risk of renal disease (50% CV disease at baseline, 59% eGFR <60 mL/min/1.73 m2). A significant 30% reduction in the cardiorenal composite primary endpoint was discussed in the Journal previously.
The pre-specified CV secondary endpoints in those with albuminuria were presented and discussed at ADA. There was:
- 20% significant RRR in 3-point MACE (CV death, MI or stroke) in those treated with canagliflozin 100 mg compared to placebo.
- 31% significant RRR in composite of CV death/HHF driven by a significant 39% reduction in HHF.
- CV death was not significantly reduced.
Cardiovascular data
Canagliflozin was demonstrated to reduce MACE and renal outcomes in a broad spectrum of sub-groups, including those with and without CVD at baseline. Presenting the data, Professor Bernard Zinman concluded that canagliflozin could be effectively used for primary and secondary prevention of major CV and renal events in people with type 2 diabetes and chronic kidney disease. Guideline and licensing changes to support this are not yet in place.
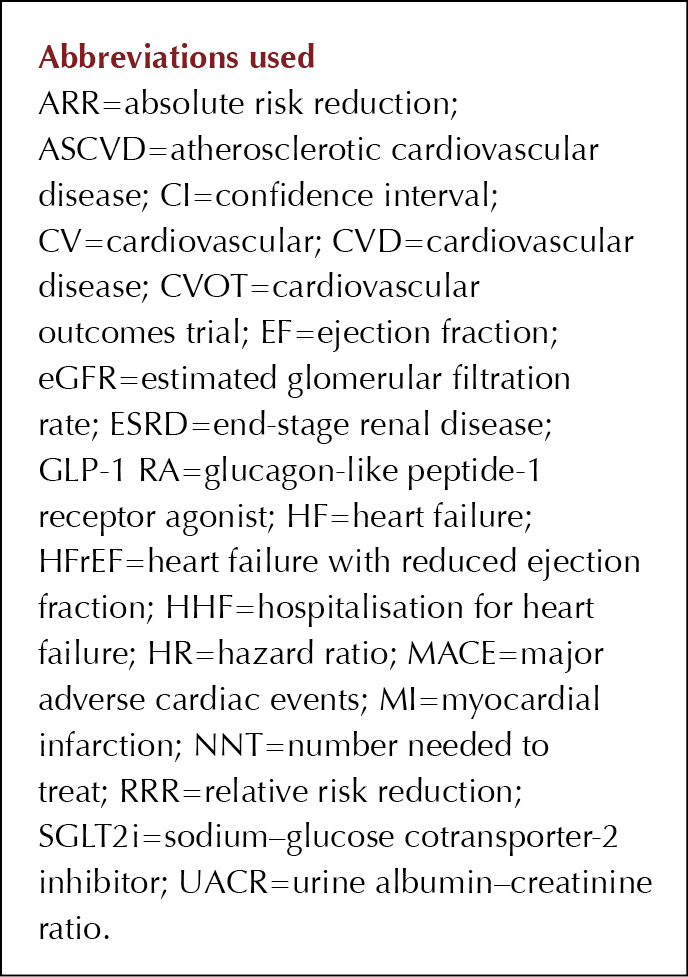
Summaries of the CV and renal outcomes by primary and secondary CV prevention cohorts are shown in Table 1.
The primary composite renal and CV endpoint data from CREDENCE were published previously (Perkovic et al, 2019).
There were seven pre-specified secondary outcomes, including 3-point MACE, the composite of CV death and HHF, and HHF alone.
Limitations
The study was stopped early at the recommendation of the independent monitoring panel after a pre-planned interim analysis once 405 events were achieved, which demonstrated that the primary outcome had been met. As the trial was stopped early, this may have impacted the power for some of the secondary outcomes that were not significant (e.g. CV mortality rate). People with an eGFR <30 mL/min/1.73 m2 were excluded from the study and, therefore, it is not known whether the findings can be generalised to this population.




SURMOUNT-5 trial pits tirzepatide against semaglutide, plus behaviour change support, for weight loss.
12 May 2025