| Take-home messages |
| ● Orlistat can effectively lower total body weight, reduce BMI, lower lipid levels and attenuate waist circumference. ● It has also been shown to cause a modest decrease in blood pressure and improved glycaemic control in people with type 2 diabetes. ● Orlistat, along with healthy lifestyle changes, helps in achieving weight loss goals, which in turn reduces obesity-related complications. |
What is orlistat?
Orlistat (Xenical®) is the saturated derivative of lipstatin, a potent natural inhibitor of pancreatic lipases, isolated from the bacterium Streptomyces toxytricini.2
Orlistat is used to manage obesity by reversibly inhibiting gastric and pancreatic lipases within the gut. These lipases play a crucial role in the digestion of dietary fat by breaking down triglycerides into absorbable free fatty acids and monoglycerides. Orlistat inhibits dietary fat absorption by approximately 30% at the recommended dosage.3
Principal effects
● The maximum benefit of orlistat is achieved when it is combined with a balanced diet and regular exercise. Weight reduction typically begins within 2 weeks of initiating orlistat. Statistically significant weight loss is observed after orlistat use exceeds 2 months.4
- Weight loss in conjunction with calorie-deficit diet and exercise at 1 year: 10.2% with orlistat vs 6.1% with placebo.5
- At 2 years, in conjunction with a weight-maintenance diet, weight regain was around 2% vs 4% in orlistat vs placebo recipients.
● Small reductions in total cholesterol, LDL cholesterol, LDL/HDL ratio, and glucose and insulin levels also occur, to a greater extent than with placebo.5
● In participants with obesity but without type 2 diabetes, orlistat plus lifestyle changes resulted in a greater reduction in the incidence of type 2 diabetes over 4 years and produced greater weight loss than lifestyle intervention alone.6
Licensed indications
● Orlistat is licensed for treatment of overweight or obesity, as an adjunct to a mildly hypocaloric diet, in individuals with:
- BMI ≥30 kg/m2, or:
- BMI ≥28 kg/m2 in the presence of other risk factors such as type 2 diabetes, hypertension or hypercholesterolaemia.
● Orlistat should be discontinued after 12 weeks if the person has been unable to lose at least 5% of body weight as measured at the start of therapy.
Position in NICE guidance
NICE NG246 advises that, after dietary, exercise and behavioural approaches have been started and evaluated in adults living with overweight or obesity, orlistat is an option for weight loss management, in accordance with its licensed indications.1
All medicines for weight management should be used alongside a reduced-calorie diet and increased physical activity. Make the decision to start medicines after discussing them with the person, and discussing the potential impact on their motivation. Arrange information, support and counselling on additional diet, physical activity and behavioural strategies when medicines are prescribed, and give information on patient support programmes.
Adverse effects
● Orlistat results in an increase in faecal fat as early as 24–48 hours after dosing. Upon discontinuation of therapy, faecal fat content usually returns to pre-treatment levels, within 48–72 hours.
● Adverse reactions to orlistat are largely gastrointestinal in nature. Incidence tends to decrease with prolonged use.
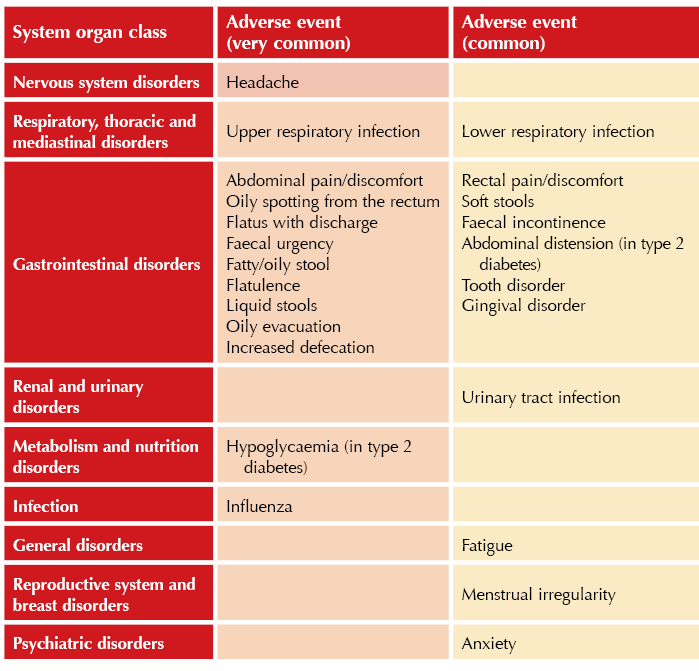
● These and other common (≥1 event per 100 users) and very common (≥1 event per 10 users) adverse effects are listed in the Table below.
● According to a scientific statement from the American Heart Association in 2021, orlistat is deemed safe and effective for treating obesity in individuals with heart failure.7
Special warnings and precautions for use
Type 2 diabetes: Reduced weight-lowering effect in people with type 2 diabetes than those without. Glucose-lowering therapies may have to be closely monitored when taking orlistat.
Fertility and pregnancy: No clinical data on exposed pregnancies are available. Exercise caution when prescribing to pregnant women.
Breastfeeding: Contraindicated when breastfeeding.
Ciclosporin: Coadministration of orlistat with ciclosporin is not recommended.
High-fat diet: Gastrointestinal adverse reactions more common when orlistat is taken with a diet high in fat (>30% of calories from fat). If taken with a very-high-fat meal, gastrointestinal adverse reactions are more likely.
Rectal bleeding: Cases of rectal bleeding have been reported. Investigate further in case of severe and/or persistent symptoms.
Contraception: The use of an additional contraceptive method is recommended to prevent possible failure of oral contraception that could occur in case of severe diarrhoea.
Anticoagulants: Coagulation parameters should be monitored in patients treated with concomitant oral anticoagulants.
Nephropathy: Orlistat may be associated with hyperoxaluria and oxalate nephropathy, leading sometimes to renal failure. This risk is increased in people with underlying chronic kidney disease and/or volume depletion.
Hypothyroidism: Rare occurrence of hypothyroidism and/or reduced control of hypothyroidism may occur, possibly due to absorption of iodine salts and/or levothyroxine.
Antiepileptics: Orlistat may decrease absorption, leading to convulsions.
Antiretrovirals for HIV: Orlistat may decrease absorption and could negatively affect the efficacy of antiretroviral medications.
Using orlistat in practice
When to use
● Clinicians are aware of the restricted criteria currently in place for use of injectable incretin therapy medications. In such cases, orlistat offers an alternative to support patients with weight management.
● In the author’s opinion, given current NHS England limitations, orlistat is an ideal choice for patient groups with BMI 30–35 kg/m2 (or BMI 28.0 kg/m2 plus comorbidities). It can also be used for higher BMIs.
● As an oral medication, orlistat may be preferable to some people who prefer not to self-inject and are willing to accept the potential adverse effects associated with this medicine.
● Note that in older people, a slightly higher BMI can have a protective effect (for example, reducing the risk of all-cause mortality). Be aware of this when treating this group of patients.1
Before initiation
● At initial assessment, take a full medical history, including concomitant medicines and weight-related comorbidities. Check that the person is willing to engage with a reduced-calorie diet and increased physical activity.
● Weight loss goals: Discuss realistic and safe weight loss goals, taking into account the person’s comorbidities and risks.
- Consider comorbidities that may improve, as well as any personal goals the person has discussed with you (such as having more energy to do the things they enjoy or to find it easier to undertake personal care).
- Weight loss of 0.5 kg to 1 kg per week is generally considered to be safe and sustainable, but tailor this to the person.
- People with type 2 diabetes may lose weight at a slower rate than people without the condition.
Posology and method of administration
● Adults: The recommended dose of orlistat is one 120 mg capsule taken with water immediately before, during or up to one hour after each main meal.
- If a meal is missed or contains no fat, the dose of orlistat should be omitted.
● The person should be on a nutritionally balanced, mildly hypocaloric diet that contains approximately 30% of calories from fat, and rich in fruit and vegetables.
● The daily intake of fat, carbohydrate and protein should be distributed over three main meals.
- Doses of orlistat above 120 mg three times daily have not been shown to provide additional benefit.
Reviewing orlistat medication
● It is recommended that orlistat is monitored regularly (ideally monthly) and that diet and lifestyle changes are reinforced at each review.
● Continue treatment beyond 3 months only if people achieve at least 5% loss from their initial body weight during the treatment.
- Less strict goals may be considered for those with type 2 diabetes.
● Treatment can continue past 12 months for weight maintenance (only after discussing benefits, risks and limitations).
Stopping orlistat
● Discontinue if weight loss is <5% after the first 12 weeks or if the person regains weight at any time whilst receiving drug treatment.
- Less strict goals may be considered for those with type 2 diabetes.
● If treatment is withdrawn, offer alternative support such as reviews at the practice or other local groups which patients may wish to use for support.





3-point MACE occurred in 12.2% vs 13.1% of tirzepatide and dulaglutide recipients, respectively, confirming non-inferiority.
19 Jan 2026