The understanding of sleep and the reasons for it is mainly a consequence of what happens when people do not sleep. Rats deprived of sleep die within 13–20 days – faster than if they are deprived of food and water but sleeping normally (Rechtschaffen et al, 1995). In humans, there is a very rare degenerative brain disease called familial fatal insomnia that can cause death within several months, but it is not known whether this is due to lack of sleep or other aspects of the disease. It is actually quite difficult for humans to achieve total sleep deprivation because of the natural drive for sleep.
When people do not get enough sleep they do not get sufficient rest and, as a consequence, do not adequately restore or conserve energy. Ultimately, this results in cognitive decline, impaired memory consolidation and decreased learning processes. Insufficient sleep also affects mood regulation, causing problems such as depression, moodiness and even increased suicidal tendencies in those who are susceptible (Neckelman, 2007). In the long term, biological and biochemical processes become adversely affected, resulting in abnormal metabolic and cardiovascular function.
Links: type 2 diabetes, obesity and sleep
Numerous studies suggest that sleep restriction has metabolic effects that predispose people to gain weight and possibly develop type 2 diabetes. Adults who sleep <5 hours per night have a 60% increased risk of becoming obese when compared to adults who sleep for longer (Cappuccio et al, 1995). The situation is similar in young people: children who sleep <10 hours per night are twice as likely to be obese as those who sleep the required 10 hours (Cappuccio et al, 1995).
Sleep duration is independently correlated with the development of type 2 diabetes. Fewer than 7–8 hours of sleep per night and excessively long periods of sleep can increase the risk of developing type 2 diabetes (Cappuccio et al, 2008). The Nurses’ Health Study reported a two-fold increase in the risk of developing type 2 diabetes if participants regularly snored at baseline (Patel et al, 2006). It also found that short sleep duration was associated with a modest increase in future weight gain and incidence of obesity, even after adjustments for other factors. Women who slept for ≤5 hours were 32% more likely to put on ≥15 kg over the 15-year study period (Patel et al, 2006).
Pathology
Obstructive sleep apnoea (OSA) occurs when the upper airway repeatedly collapses during sleep, causing breathing to stop (apnoea) or become inadequate (hypopnoea; Figure 1). The muscles maintain upper airway patency. With sleep onset this compensatory mechanism is decreased, which may contribute to airway narrowing or collapse and lead to the production of noise when air is forced through the airway.
Reduced or absent airflow results in hypercapnia and hypoxia, which increases the patient’s breathing effort against the collapsed airway. The patient is aroused and hyperventilates to increase oxygen and decrease carbon dioxide to normal levels before returning to a deeper stage of sleep. The cycle then begins again.
The hypoxia, hypercapnia and increased ventilatory effort activate the sympathetic nervous system, resulting in increased heart rate, constriction of the peripheral blood vessels and increased mental activity. This may occur with each OSA event. Increased mental activity may cause the sleep fragmentation often seen in OSA, resulting in excessive daytime sleepiness. Chronic activation of the sympathetic nervous system may lead to and/or worsen cardiac conditions.
When the physiological condition of OSA is associated with the symptom of excessive daytime sleepiness, this is termed OSA syndrome.

Effects on the body
There is a high prevalence and wide spectrum of undiagnosed sleep apnoea in the general population. OSA is a chronic life-altering disease. It results in unrefreshing sleep and/or morning headaches as well as excessive daytime fatigue. In the short-term, performance is reduced. OSA may result in impaired attention and concentration, hence its association with road traffic accidents (see Capehorn, 2018).
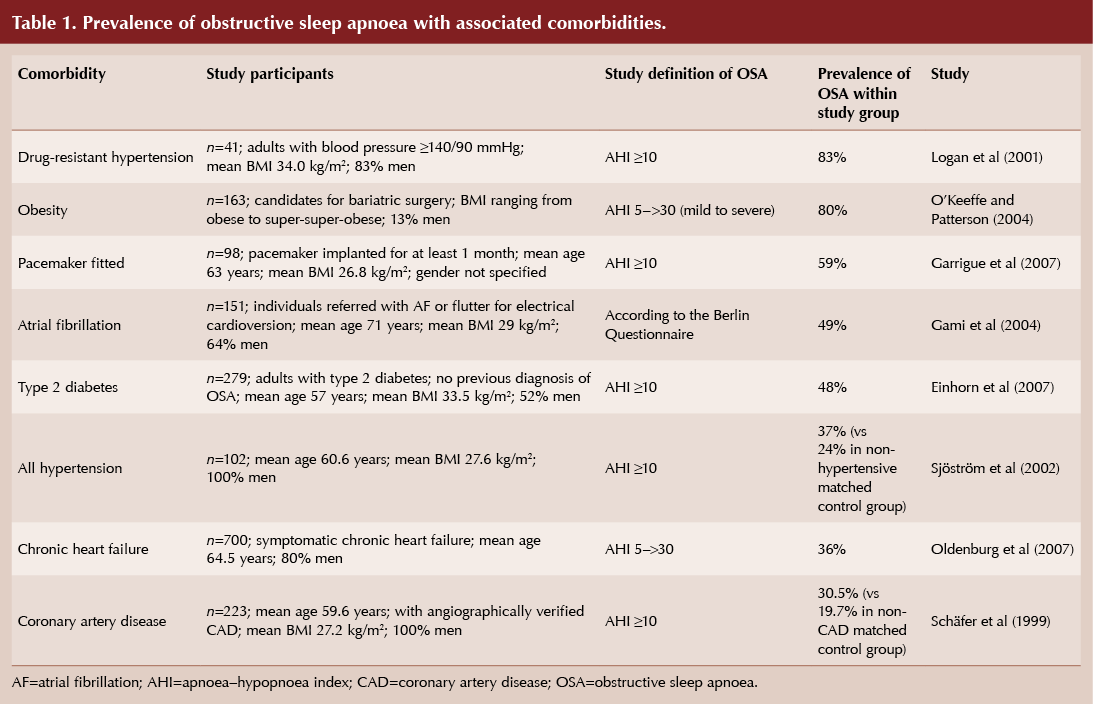
Even mild sleep apnoea is associated with significant potential health consequences. In the long-term sleep apnoea can adversely affect blood pressure, glucose tolerance and the risk of cardiovascular events (Table 1). The pathophysiological links between OSA and carbohydrate metabolism disorders are detailed in the International Diabetes Federation (IDF) consensus statement (IDF, 2008). In addition to OSA’s association with comorbidities, it also confers a higher risk of all-cause and cardiovascular mortality (Young et al, 2008).
Predisposing factors
There are a number of factors that predispose individuals to the development of OSA.
There is a bi-directional relationship between obesity and OSA: obesity and weight gain can increase the severity of OSA and, conversely, weight reduction can decrease severity (Adult Obstructive Sleep Apnoea Task Force, 2009). Although not all people with OSA are overweight, obesity (BMI ≥30) is a major predisposing factor for OSA and the coexistence of obesity and diabetes has a cumulative effect on the prevalence of OSA. It has also been proposed that OSA can cause additional weight gain, with changes noted in leptin levels and other gut hormones that have an effect on adipocytes. These changes make individuals with OSA more susceptible to increased caloric intake, predisposing them to weight gain, independent of the increased risk of weight gain due to daytime tiredness (Adult Obstructive Sleep Apnoea Task Force, 2009).
Anatomic abnormalities, such as a receding chin or enlarged tonsils, can be associated with an increased likelihood of OSA, as they make a person more susceptible to upper airway collapse. This is especially important to keep in mind if the person is of normal body weight. An increased neck circumference (>17 inches in men, or >16 inches in women) is an independent predisposing factor (Adult Obstructive Sleep Apnea Task Force, 2009).
Alcohol or sedative use will affect breathing, as both change the muscle tone of the upper airway. Ironically, patients with OSA often present with fatigue or sleep disorders and are treated with sedatives, sedative antidepressants or low-dose tricyclic antidepressants, which make the situation worse.
OSA is more common in men, but its prevalence increases with age (presumably as a consequence of decrease in muscle tone) and there is a familial relationship. Asian ethnicity and having first-degree relatives with OSA have been linked with increased risk of OSA (Seicean et al, 2008). Smoking increases the likelihood of OSA as it causes inflammation of the upper airway (Krishnan et al, 2014). There are also possible associations with asthma (Teodorescu et al, 2015).
You should consider the possibility of OSA in patients presenting with:
- Fatigue or unrefreshing sleep
- Morning headaches
- Hypertension (newly-diagnosed as well as resistant/refractory)
- Congestive heart failure
- Bariatric surgery
- Large neck size
- Small jaws or micrognathia
- Metabolic syndrome
- Memory loss and concentration issues
- Personality changes, such as irritability or depression
- Sexual dysfunction.
The most common symptom of OSA is snoring; however, there are people who snore but still breathe regularly without sleep disruption. A bed partner or family member often knows there is a problem before the person with OSA, as they are likely to witness the snoring, pauses in breathing and gasping for breath.
Case finding
There are a number of OSA case finding tools available to GPs. OSA questionnaires assess sleep habits and subjectively assess whether people wake feeling unrefreshed. Examples include:
- Epworth Sleepiness Scale:
www.britishsnoring.co.uk/sleep_apnoea/epworth_sleepiness_scale.php - Berlin Questionnaire: www.britishsnoring.co.uk/berlin_questionnaire.php
- STOP-BANG Questionnaire:
www.stopbang.ca/osa/screening.php
These tools are quick to fill out and are freely available online. As such, they are useful for making an initial assessment in the primary care setting.
The Epworth sleepiness scale (Foster et al, 2009) includes eight everyday situations and asks the person what their chance of dozing is. Each situation is scored from 0 to 3. A total score >10 indicates the presence of significant daytime sleepiness and is indicative of an underlying medical condition that should be investigated.
The Berlin questionnaire (British Snoring and Sleep Apnoea Association, 2018) consists of three categories and risk is based on patient responses to individual items and the overall scores in the symptom categories. The STOP-BANG questionnaire (University of Toronto, 2012) asks yes/no questions relating to Snoring, being Tired, being Observed to stop breathing or choke/gasp, high blood Pressure, BMI, Age, Neck size and Gender. Patients are at low risk of OSA if they answer yes to 0–2 questions and high risk if they answer yes to ≥5 questions. They are also at high risk if they answer yes to two or more of the four STOP questions and are male or have a BMI >35 or a neck circumference of ≥43 cm (male) or ≥41 cm (female).
Based on questionnaire results, patients can be provided with lifestyle advice on sleep hygiene, losing weight and smoking cessation. If it is suspected that the patient has OSA, he or she should be referred to a sleep clinic or assessed in the community. OSA is a potentially fatal condition and, although sleep clinics have limited capacity, an effort must be made to identify every case.
Portable sleep study devices
There are an increasing number of specific devices to monitor individuals’ sleep overnight, including ones designed for use in the community. Portable monitoring for the diagnosis of OSA in obese patients displays good sensitivity and specificity (Oliveria et al, 2015).
The Rotherham Institute for Obesity used ApneaLink Air to case find obese patients with symptoms of sleep problems, a raised neck circumference or a diagnosis of type 2 diabetes. Of 233 patients screened by this primary care-based programme, 167 (72%) had an apnoea–hypopnoea index (AHI) >5, indicating at least mild OSA (see Capehorn, 2018). The ApneaLink Air results showed good correlation with subsequent secondary care testing. Of the 111 patients assessed in secondary care at the end of the study, 71 (64%) had been prescribed continuous positive airway pressure (CPAP) to treat their OSA, 27 had milder symptoms and were encouraged to lose weight, and 13 were found to not have OSA. Eighty-four participants were obese and had type 2 diabetes. Of the 44 with an AHI >5, 25 were prescribed CPAP and nine were encouraged to lose weight.
Treatment
Patients with OSA should be assessed and treated by a specialist in sleep disorders (NICE, 2008). There are a number of treatments available, depending on severity:
- Dental appliances (mild OSA).
- Behaviour modification: weight loss if obese, smoking cessation, limiting alcohol consumption, avoiding sedatives and sleeping tablets, and sleep hygiene techniques (mild or positional OSA).
- Surgery: tonsillectomy, adenoidectomy, genioglossal or maxillomandibular advancement or uvulopalatopharyngoplasty if clinically indicated.
- Mechanical: continuous positive airway pressure (CPAP).
Weight loss (>10%) reduces the severity of OSA symptoms and AHI but is difficult to achieve and maintain without the help of specialist weight management services, anti-obesity pharmacotherapy or bariatric surgery (Adult Obstructive Sleep Apnoea Task Force, 2009). Interestingly very low calorie diets can lead to effective weight loss that will improve OSA in the majority of patients and initial improvements can be maintained beyond 1 year (Johannson et al, 2009; 2011).
Raising the issue of sleep hygiene in relation to its duration and quality may be beneficial if appropriate advice is given in a “brief intervention”.
CPAP is recommended by NICE (2008) as a possible treatment for adults with moderate or severe OSA. The raised air pressure prevents the patient’s airway closing or narrowing during sleep. The level of positive airway pressure should be determined for each patient during a sleep study. CPAP treatment aims to maintain an open airway during inspiration and exhalation, improve sleep quality and reduce daytime symptoms associated with OSA.
The level of adherence with therapy has an impact on the clinical benefit of CPAP (Kribbs et al, 1993). GPs can encourage adherence by regular checking, follow-up with the specialists and by reminding people of the implications of not following the treatment, which include the effects on comorbidities and safety to drive.
OSA and the consequential daytime sleepiness significantly increases the likelihood of road traffic accidents. If an individual is confirmed as having OSA that requires treatment and that person is adherent to that treatment, it will not affect their ability to drive. However, if the individual does not adhere to their treatment, the DVLA should be informed and that individual should not drive.
Summary
OSA is a common disorder that often coexists with diabetes. Fragmented sleep and the resulting hypoxia are thought to play a role in the association between OSA and cardiovascular disease and metabolic dysfunction. GPs should carry out an initial assessment of diabetes patients who present with factors predisposing them to OSA, such as obesity, or symptoms of OSA, such as daytime sleepiness. Those with suspected OSA should be referred to a specialist sleep service.





Risk ratios of 1.25 for autism spectrum disorder and 1.30 for ADHD observed in offspring of mothers with diabetes in pregnancy.
18 Jun 2025