Abbreviations used
Trials:
DAPA-HF = Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DPPOS = Diabetes Prevention Program Outcomes Study; VERTIS CV = Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes
ACR = albumin to creatinine ratio; CI = confidence interval; CV = cardiovascular; CVOT = cardiovascular outcomes trial; DKA = diabetic ketoacidosis; eGFR = estimated glomerular filtration rate; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HHF = hospitalisation for heart failure; HR = hazard ratio; IGT = impaired glucose tolerance; MACE = major adverse cardiovascular event; MI = myocardial infarction; NDH = non-diabetic hyperglycaemia; NT-proBNP = N-terminal pro B-type natriuretic peptide; RAAS = renin–angiotensin–aldosterone system; SGLT2i = sodium–glucose cotransporter-2 inhibitor
Ertugliflozin cardiovascular safety
Ertugliflozin, an SGLT2i, does not increase the risk of MACE (CV death or non-fatal MI or non-fatal stroke) in people with type 2 diabetes and established atherosclerotic CV disease, but the drug failed to show superiority over placebo, according to the VERTIS CV study results presented at the American Diabetes Association’s 80th Scientific Sessions.
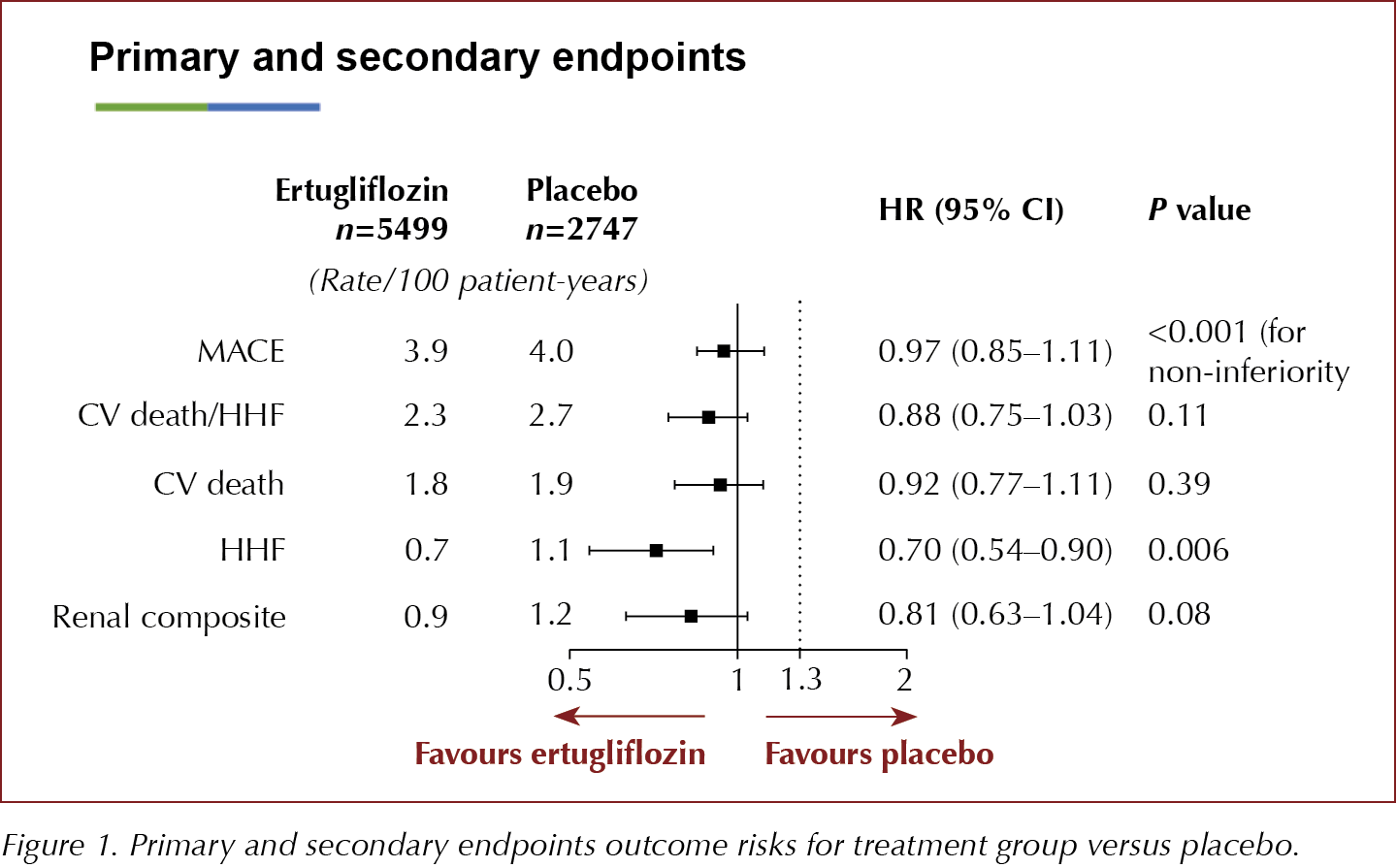
The key secondary composite endpoint of risk of CV death/HHF was not significantly decreased, but there was a 30% reduction in the rate of HHF amongst those treated with ertugliflozin. The renal composite outcome was nearly 20% lower with ertugliflozin than with placebo, but this was not significant. The study authors concluded that the effects on HHF and renal outcomes were in line with those seen in the other CVOTs with SGLT2is.
8246 people aged ≥40 years with established atherosclerotic disease (MI, stroke or peripheral arterial disease) were randomised 1:1:1 to receive ertugliflozin 5 mg or 15 mg or placebo and followed for up to 6.1 years, to assess time to the first MACE (CV death, non-fatal MI or non-fatal stroke). The two ertugliflozin groups were combined for the analysis and compared to placebo. The primary outcome of 3-point MACE occurred in 11.9% of participants in both the ertugliflozin-treated and the placebo groups, demonstrating no difference in CV risk between the groups, with an HR of 0.91 (95.6% CI, 0.77–1.07) for the ertugliflozin 5 mg group and 1.04 (95.6% CI, 0.89–1.21) for the 15-mg group versus placebo.
Secondary endpoints and results were:
- CV death/HHF: HR, 0.88 (95.8% CI, 0.75–1.03)
- CV death alone: HR, 0.92 (95.8% CI, 0.77–1.11)
- HHF alone: HR, 0.70 (95% CI, 0.54–0.90)
- Renal composite outcome (renal death, dialysis, transplant or doubling of serum creatinine): HR, 0.81 (95.8% CI, 0.75–1.03).
The secondary endpoint of CV death and HHF narrowly missed significance and, therefore, due to the hierarchical significance testing, the 30% reduction in the HHF must be considered to be exploratory only. The 19% reduction in the renal composite was not significant. Changes in eGFR over time demonstrated a just over 3 mL/min/1.73 m2 difference between both ertugliflozin groups compared to placebo at 60 months.
The safety profile was described as comparable to that of other SGLT2is, including low frequency of amputations and DKA, but numerically more in those treated with ertugliflozin than placebo (amputations, 0.6 v 0.5 per 100 patient-years, respectively; risk difference, 0.1 [95% CI, −0.1–0.30]). As anticipated, there was a statistically increased risk of urinary tract infections and genital mycotic infections in the ertugliflozin-treated groups compared to those receiving placebo and no cases of Fournier’s gangrene. There were no unanticipated adverse events, and comparable levels of hypoglycaemia, hypovolaemia-related events and acute pancreatitis in the two groups.
The groups were well matched at baseline, with mean age just under 65 years; 70% were male and 88% were white. All people recruited had CV disease, and 22% had eGFR <60 mL/min/1.73 m2 at baseline. Participants had a mean duration of type 2 diabetes of 13 years, and an HbA1c of just over 8% (64 mmol/mol) and mean BMI in the obese range. Two thirds were on two or more glucose-lowering drugs at baseline, and more than 80% were on RAAS blockers and a statin, with 15% on a loop diuretic and 43% on any diuretic. In March 2016, following the publication of the EMPA-REG study results there was a protocol amendment to VERTIS CV to double the sample size and add efficacy objectives for superiority of CV and renal outcomes.
During the symposium, speakers sought to put the VERTIS CV results into context with the other SGLT2i CVOTs, comparing baseline CV risk, and CV and renal outcomes between studies. They also shared a new unpublished meta-analysis of the SGLT2i CVOTs, incorporating data from VERTIS CV. Time to first HHF comparisons are shown in Figure 3.
Ertugliflozin currently costs 20% less than other SGLT2 inhibitors in the UK.


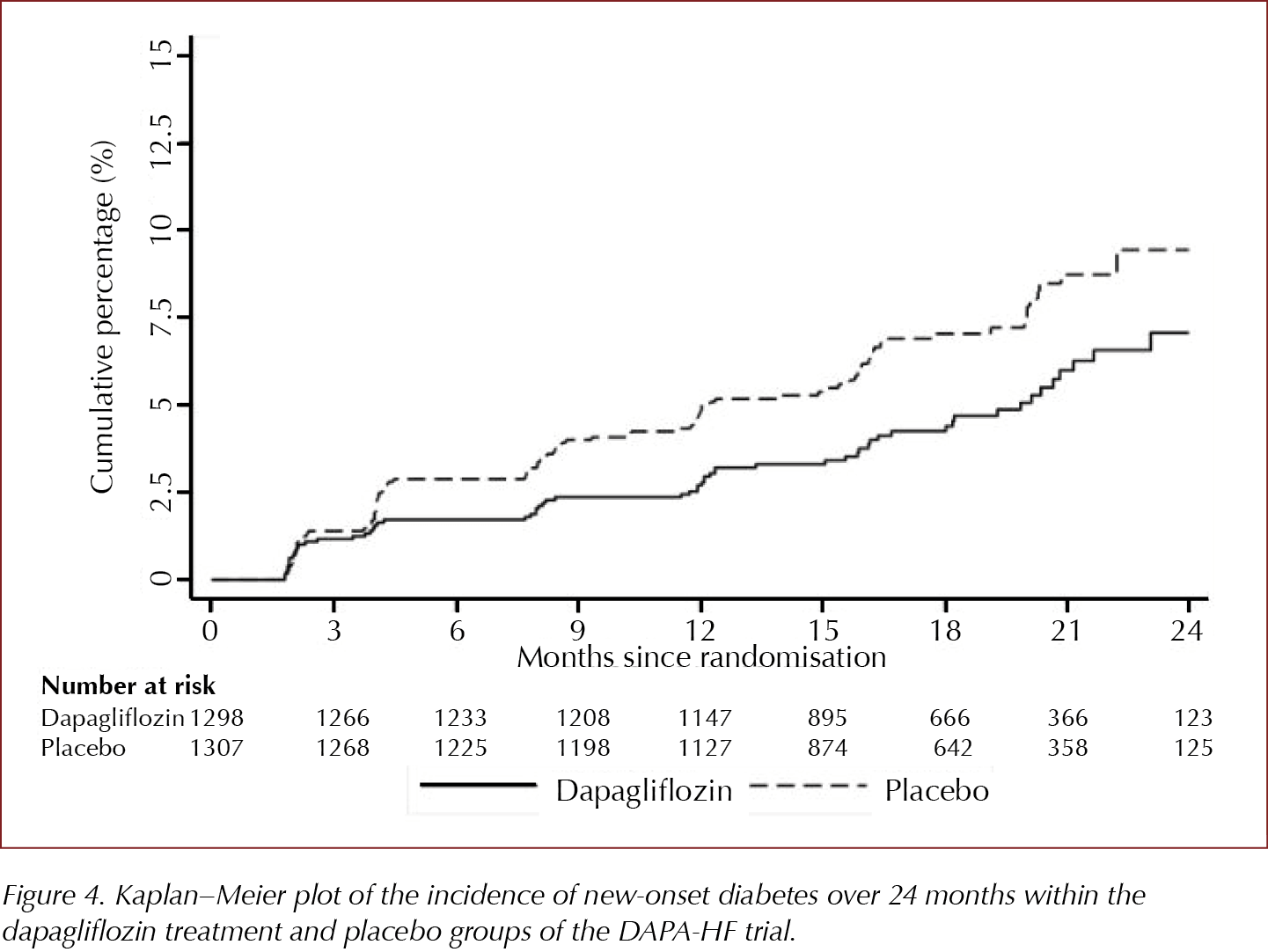
Dapagliflozin may help prevent type 2 diabetes according to DAPA-HF trial
Amongst those without type 2 diabetes at baseline in the DAPA-HF trial, in a pre-planned subgroup analysis, there was a 32% reduction in development of type 2 diabetes in those randomised to dapagliflozin 10 mg versus those treated with placebo. Those aged ≤65 years and those with lower than median levels of NT-proBNP experienced most preventive benefit.
The DAPA-HF trial recruited people with HF with reduced ejection fraction (HFrEF) with and without type 2 diabetes and demonstrated a significant reduction versus placebo in its composite primary outcome of worsening HF and cardiovascular death. An exploratory pre-planned subgroup analysis demonstrated no difference in the primary or secondary outcomes in the subgroups with type 2 diabetes, non-diabetic hyperglycaemia or normal glycaemia (HbA1c <5.7% [39 mmol/mol]).
55% (2605) of those recruited to the trial did not have type 2 diabetes at baseline. In this pre-planned subgroup analysis presented at the ADA’s 80th Scientific Sessions, 4.9% of those treated with dapagliflozin 10 mg compared to 7.1% of those in the placebo-treated group developed type 2 diabetes, an HR of 0.68 (95% CI, 0.50–0.94). This 32% reduction in development of type 2 diabetes was similar to the 31% reduction seen in the Diabetes Prevention Program study in those treated with metformin (Knowler et al, 2002; Aroda et al, 2017).
157 trial participants developed type 2 diabetes and 150 (95%) of them had non-diabetic hyperglycaemia (NDH)/pre-diabetes at baseline. NDH was defined using the US criterion as HbA1c 5.7–6.4% (39–47 mmol/mol). If the UK criterion of 6.0–6.4% (42–47 mmol/mol) was used, then 86.6% of those developing type 2 diabetes during the trial had NDH at baseline. At baseline, on average the group who developed type 2 diabetes was slightly older (around 3 years), had higher HbA1c levels as expected, slightly higher BMI and lower eGFR levels compared with those who did not develop type 2 diabetes.
Dapagliflozin is not currently licensed for type 2 diabetes prevention.
DPPOS demonstrates persistent reduction in type 2 diabetes after 22 years
The Diabetes Prevention Program Outcomes Study (DPPOS) demonstrates continued significant reduction in the risk of developing type 2 diabetes after average 22-year follow-up, with a 25% reduction in those in the lifestyle group and 18% reduction in those treated with metformin compared to the original placebo group. The risk reduction from lifestyle has declined over the years, whereas the benefit from metformin has persisted at 18% since the 10-year data. People who did not develop type 2 diabetes had a significant 39% reduction in MACE, 57% reduction in early retinopathy and 37% reduction in nephropathy when compared to those who developed type 2 diabetes. When lifestyle or metformin effects were evaluated separately, neither demonstrated a significant reduction in CV disease, retinopathy or nephropathy, but long-term metformin demonstrated a trend towards reduction in stroke and a trend towards reduction in CV events amongst those who started metformin aged <45 years. There was a non-significant 12% reduction in cancer risk associated with metformin use.
In the original Diabetes Prevention Program (DPP) involving 3234 people with IGT, over an average of 3 years there was a 58% reduction in development of type 2 diabetes with lifestyle and 31% reduction with metformin compared to the placebo group. Lifestyle was particularly effective (71% reduced risk) in those aged ≥60 years, while metformin was particularly effective in younger people, those with higher BMI or previous gestational diabetes.
At the end of the DPP, all three groups were offered group-implemented lifestyle intervention in view of the benefits identified during the study; 88% (both those with and without type 2 diabetes) agreed to participate in the DPPOS and continue with monitoring. DPPOS was designed to determine the longer-term impact of the previous intensive lifestyle intervention or metformin on type 2 diabetes development and the development of complications, such as retinopathy, nephropathy and CV complications. People in the original metformin group continued on treatment, allowing evaluation of long-term effects of the drug on outcomes, including CV disease and cancer. As the population in the study age (current average age is 72 years), the impact of the interventions on frailty measures is being explored. DPPOS is the longest duration and largest prevention study, and continues to follow around 75% of the original participants from the DPP who remain alive.
CV disease
Pre-diabetes/IGT has been shown to be associated with increased risk of CV disease even before type 2 diabetes develops. In the DPPOS, the primary outcome is the first occurrence of 3-point MACE (CV death, non-fatal MI and non-fatal stroke) and this was 39% lower in those who did not develop type 2 diabetes compared to those who did. Surprisingly, however, there was no significant beneficial effect of either metformin or lifestyle on CV outcomes.
Nephropathy
In the DPPOS, the primary nephropathy outcome is a composite of development of eGFR <45 mL/min/1.73 m2 or ACR ≥30 mg/g. This demonstrated a significant reduction in those who did not develop type 2 diabetes compared to those who did, as did the development of the secondary outcome of ACR ≥30 mg/g alone.
Amongst the oldest group of participants, there was a modest increased risk of nephropathy in those treated with long-term metformin, which was the only negative finding in the study.
Retinopathy
Original treatment group assignment did not change the risk of developing retinopathy, which was defined as the presence of microaneurysms, exudates or haemorrhages in either eye on fundus photographs. Retinopathy occurred in some people with pre-diabetes, and increased in those who developed type 2 diabetes compared with those who did not. Amongst those who developed type 2 diabetes, higher HbA1c was associated with increased retinopathy.
Cancer
A previous meta-analysis had suggested a reduction in cancer incidence, but not cancer mortality, with treatment with metformin (Gandini et al, 2014). In the 22-year DPPOS data, metformin use was associated with only a non-significant 12% lower risk of cancer.





The mortality benefits of smoking cessation may be greater and accrue more rapidly than previously understood.
2 Apr 2024