Update history
11th July 2023: First published.
18th July 2023: Updated to include NICE TAs on dapagliflozin and empagliflozin for chronic heart failure in algorithm.
17th October 2023: Updated to reflect changes in licence of empagliflozin.
20th October 2023: Updated to clarify licence of canagliflozin for treatment of DKD in people with type 2 diabetes.
22nd July 2025: Updated to reflect new NICE TAs on dapagliflozin/empagliflozin for CKD/HFpEF in algorithm.
What and why
● SGLT2 inhibitors (SGLT2is) are oral drugs that block reabsorption of glucose in the kidneys.
● SGLT2is were initially developed as glucose-lowering drugs and were noted to have beneficial effects on systolic BP and weight reduction. The cardiovascular outcome trials and studies in people with renal disease and heart failure confirm significant benefits in these comorbidities, which appear to be independent of glucose lowering, with the HF and CKD benefits demonstrated in people with and without T2DM.
● SGLT2is can be used at most stages of the T2DM pathway but remain underused in the UK, depriving people with T2DM of the added benefits for ASCVD, HF, CKD, weight loss and BP lowering.
● When prescribing SGLT2is, consider safety and tolerability.
● Although SGLT2is do not increase risk of hypoglycaemia, if adding an SGLT2i to insulin or a sulfonylurea, initially reduce the dose of insulin or SU to reduce risk of hypoglycaemia.
When to use SGLT2 inhibitors in type 2 diabetes
Many guidelines and guidance documents have informed the positioning of SGLT2 inhibitors since the previous version of this document was published. These include the 2022 update to NICE NG28 and the 2022 updated ADA/EASD consensus on glycaemic management.
These guidelines differ in their positioning of GLP-1 receptor agonists (GLP-1 RAs) since NICE did not revisit the evidence base for GLP-1 RA use for glucose lowering and did not find them cost-effective as a class for atherosclerotic cardiovascular disease (ASCVD) risk reduction due to differing cardiovascular benefit between drugs. Since advice on SGLT2i use is now similar between NICE and ADA/EASD guidelines, this article will focus on the 2022 update of NICE NG28.
As detailed in the Algorithm below, NICE recommends the use of an SGLT2i as dual therapy with metformin (or alone if metformin is contraindicated or not tolerated) in people with established or high risk of ASCVD, and in combination with ACE inhibitors or ARBs in people with chronic kidney disease (CKD) or diabetic kidney disease (DKD).
SGLT2is are also options for monotherapy in some individuals who are not at high ASCVD risk, and as dual or triple therapy when other agents fail to control HbA1c below the individual’s agreed target.
Significant glucose lowering is unlikely if eGFR is <45 mL/min/1.73 m2, but other benefits in terms of heart failure, slowing CKD progression and ASCVD protection persist. For more details of specific licensed indications and eGFR thresholds for initiation and stopping, see our Need to know guide and the SmPCs for individual drugs.
Before starting an SGLT2i, check whether the person is at increased risk of diabetic ketoacidosis (DKA), address modifiable risks for DKA, check whether suitable for an SGLT2i (see below), and fully counsel regarding sick day guidance (see below).
Safe use: SGLT2is and renal function
● Check electrolytes and eGFR prior to therapy; monitor annually unless eGFR is <60 mL/min/1.73 m2, when eGFR should be checked every 3–6 months (see NICE NG203 guidance).
● Modest reductions in eGFR may occur when starting SGLT2is, but extra monitoring is not required unless unwell or starting another drug likely to impact renal function. eGFR will improve and, over the longer term, SGLT2is slow progression of CKD.
● See our Need to know guide for eGFR limits on initiating and stopping individual drugs for specific indications, as this is now complex.
● Dapagliflozin and empagliflozin are licensed for use in chronic symptomatic HFrEF and HFpEF.
● Canagliflozin is licensed to slow progression of DKD and dapagliflozin is licensed to slow progression of DKD and CKD in people with and without type 2 diabetes. Empagliflozin has evidence of a renoprotective benefit, and this is reflected in the wording of the SmPC.
● Glucose-lowering effects are minimal when eGFR is <45 mL/min/1.73 m2, so additional glucose-lowering therapies may be needed.
● SGLT2is may increase the risk of dehydration and hypotension in those treated with thiazide or loop diuretics. Caution is required particularly in older people.
Choosing who to treat with SGLT2 inhibitors
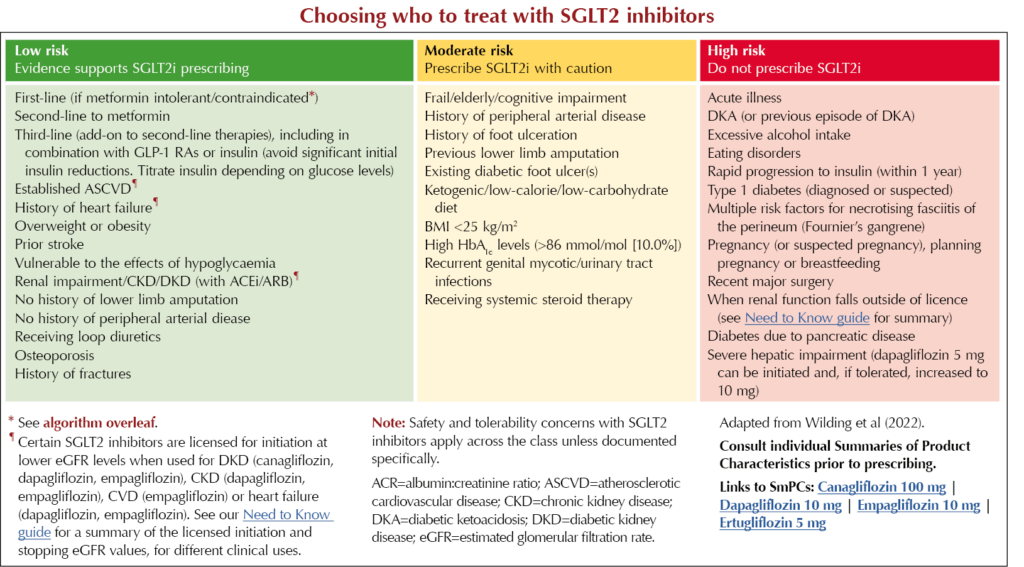
NICE guidance on SGLT2 inhibitor use in people with type 2 diabetes

Safety issues with SGLT2 inhibitors
Diabetic ketoacidosis (DKA) – see MHRA (2016)
● May be euglycaemic with only slight increases in blood glucose: <14 mmol/L. Test blood ketones even if glucose levels are normal.
● May be associated with dehydration, low food intake, weight loss, infection, surgery, vomiting, decreased insulin dose, poor glycaemic control.
● Suspect if nausea, vomiting, anorexia, abdominal pain, thirst, difficulty breathing, confusion, or unusual fatigue and sleepiness.
● Warn to seek medical care immediately if these develop. Most people will require admission.
● Pause SGLT2i drugs during acute illness and prior to surgical procedures – see Sick-day guidance below.
● Do not restart an SGLT2i if DKA occurs unless there was a clear precipitating factor.
● Pause treatment in those hospitalised for surgery or acute illness.
● Factors in history that may predispose to DKA include low beta-cell reserve, sudden reductions in insulin, increased insulin requirements due to acute illness, surgery and alcohol abuse.
● Warn against combining an SGLT2i with a very-low-carbohydrate/ketogenic diet, as these greatly increase DKA risk; people eating this way are not suitable for an SGLT2i.
Sick-day guidance for type 2 diabetes
Blood glucose can rise during illness even if the person is not eating. When ill and at risk of dehydration, people with T2DM should be advised to:
● Temporarily stop SADMANS drugs (SGLT2is, ACEis, Diuretics [individualise in those with HF], Metformin, ARBs, NSAIDs and Sulfonylureas*), and if unable to eat or drink, or persistent vomiting or diarrhoea, to contact their GP or specialist nurse for advice.
● If they are unsure how to self-manage during illness, encourage the person to contact their practice or diabetes specialist team, or seek emergency medical advice.
● If not able to eat normally, replace meals with high-carbohydrate snacks or drinks.
● Stay well hydrated (2–3 litres of fluid per day) and eat little and often.
● Advise to keep taking insulin and all other diabetes medicines (apart from SADMANS drugs) even if not eating.
● Give people taking SGLT2i drugs specific advice about the risk of euglycaemic DKA and to consult if they become ill, even if blood glucose levels are not high. Primary care teams should be aware of the need to test blood glucose AND ketones in this situation.
● See How to advise on sick day rules.
*For SUs, consider stopping if unable to eat or drink but be guided by results of self-monitoring of blood glucose.
Genital and urinary infections
● Thrush-type genital infections are common.
● Infections are more common early in treatment and when glucose levels are high (even without SGLT2is). Providing hygiene information may reduce risk and improve treatment continuation.
● Treat with topical or oral antifungals.
● Most people can continue SGLT2i treatment.
● Glycosuria may cause urinary symptoms and more frequent voiding.
● UTIs are relatively rare but urosepsis may occur; manage with standard antibiotics. If recurrent, stop SGLT2i treatment.
Fournier’s gangrene (necrotising fasciitis of the perineum) – see MHRA (2019)
● Rare but serious and potentially life-threatening. Diabetes and SGLT2i treatment are risk factors.
● May be preceded by urogenital infection or perineal abscess.
● Advise to seek urgent medical attention if severe pain, tenderness, erythema or swelling in genital area accompanied by fever or malaise.
● Usually occurs mainly in men but with SGLT2i treatment can occur in women.
Lower limb amputations – see MHRA (2017)
● Amputations are eight times more likely in those with diabetes than those without.
● Increased risk of amputation with canagliflozin in CANVAS trial programme but not in CREDENCE.
● Increased amputations across the ertugliflozin studies, notably with the 15 mg dose.
● Warnings remain on the SmPCs for these two drugs.
● No increased risk identified with empagliflozin (Inzucchi et al, 2018) or dapagliflozin (Bonaca et al, 2020).
● Proposed risk factors include previous amputation, peripheral vascular disease and neuropathy, but no specific mechanisms confirmed.
● Footcare education should be provided at initiation and during SGLT2i therapy.
● Many clinicians pause SGLT2i therapy during active foot ulceration, infection, osteomyelitis or gangrene.
● Meta-analysis of 15 randomised controlled trials involving 63 716 people with and without type 2 diabetes did not identify any increased risk of amputation with SGLT2is (See et al, 2022).
Bone fractures
● Small increased fracture risk and changes in bone mineral density (BMD) seen in those treated with canagliflozin compared with placebo in the CANVAS (but not CANVAS-R or CREDENCE) trial, and with ertugliflozin, but not identified in empagliflozin or dapagliflozin studies.
● Fractures occurred mainly early in treatment and may have been linked to increased falls due to volume depletion and hypotension.





3-point MACE occurred in 12.2% vs 13.1% of tirzepatide and dulaglutide recipients, respectively, confirming non-inferiority.
19 Jan 2026