What is tirzepatide?
Tirzepatide (trade name Mounjaro®) is a new glucose-lowering medicine in the “incretin family” which, to date, has included the glucagon-like peptide-1 (GLP-1) receptor agonists (such as dulaglutide and semaglutide) and dipeptidyl peptidase-4 (DPP-4) inhibitors (including linagliptin and sitagliptin). Tirzepatide is the first in its class, being a single-molecule agonist of both the GLP-1 receptor and the receptor for glucose-dependent insulinotropic polypeptide (GIP), hence the term “dual agonist” or “twincretin”. It is a peptide molecule with a long duration of action, allowing for once-weekly subcutaneous injection in clinical practice.
Licensed indications
In the UK, tirzepatide has two licensed indications:
1. For the treatment of adults with insufficiently controlled type 2 diabetes as an adjunct to diet and exercise:
- As monotherapy when metformin is considered inappropriate due to intolerance or contraindications, or:
- In addition to other medicinal products for the treatment of type 2 diabetes.
2. For weight management, including weight loss and weight maintenance, as an adjunct to a reduced-calorie diet and increased physical activity in adults with an initial BMI of:
- ≥30 kg/m2 (obesity), or:
- ≥27 kg/m2 to <30 kg/m2 (overweight) in the presence of at least one weight-related comorbid condition (e.g. hypertension, dyslipidaemia, obstructive sleep apnoea, cardiovascular disease, prediabetes or type 2 diabetes).
Place in NICE guidelines
To date, NICE has only considered the glucose-lowering indication for tirzepatide. The TA924 guidance1 recommends tirzepatide for treating type 2 diabetes alongside diet and exercise in adults when it is insufficiently controlled only if:
● Triple therapy with metformin and two other oral antidiabetes drugs is ineffective, not tolerated or contraindicated, and:
- BMI is ≥35 kg/m2 and there are specific psychological or other medical problems associated with obesity, or:
- BMI is <35 kg/m2, and:
- Insulin therapy would have significant occupational implications, or:
- Weight loss would benefit other significant obesity-related complications.
Lower BMI thresholds (usually reduced by 2.5 kg/m2) are recommended for people from South Asian, Chinese, other Asian, Middle Eastern, Black African or African–Caribbean family backgrounds.
This article will focus on the NICE-approved indication of tirzepatide for glucose lowering in people with type 2 diabetes.
Effects on blood glucose
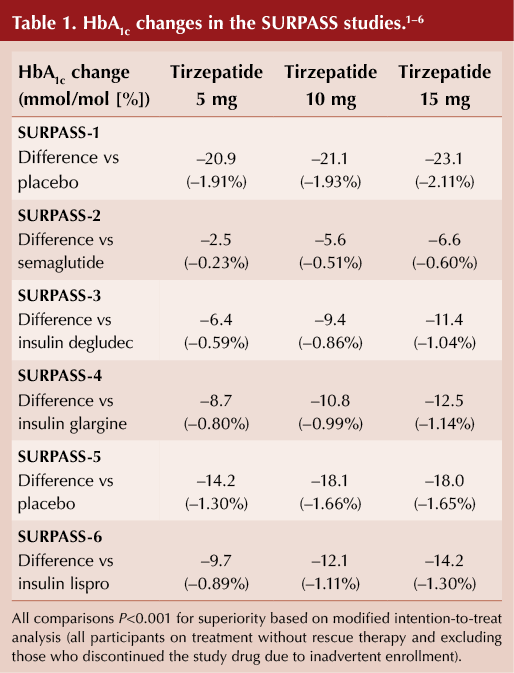
The SURPASS clinical trial programme assessed the efficacy and safety of tirzepatide as a treatment to improve glycaemic control in people with type 2 diabetes. Tirzepatide has been compared with placebo, subcutaneous semaglutide, insulin degludec, insulin glargine and insulin lispro.1–6
In people with type 2 diabetes, over follow-up of 40–50 weeks, tirzepatide 5 mg, 10 mg and 15 mg once weekly demonstrated:
- Superior HbA1c reduction from baseline versus all comparators (see Table 1).
- Clinically meaningful and significantly greater proportions of participants achieving HbA1c goals of <53 and ≤48 mmol/mol versus comparators.
Effects on weight
In the SURPASS programme, change in weight was a prespecified secondary outcome. In adults with type 2 diabetes, treatment with tirzepatide 5 mg, 10 mg and 15 mg once weekly was associated with superior weight reduction from baseline compared with placebo, semaglutide 1 mg weekly, titrated insulin degludec, titrated insulin glargine and titrated insulin lispro as an add-on to insulin glargine.1–6
Cardiovascular outcomes
The cardiovascular outcomes trial for tirzepatide, SURPASS-CVOT, is an active-comparator study versus dulaglutide 1.5 mg weekly and is ongoing. It was originally anticipated to end in mid-2024 but, as it is “event-driven”, the trial will only report when a predefined number of cardiovascular endpoints have occurred.
In accordance with US Food and Drug Administration guidelines, a meta-analysis of cardiovascular events in several phase 2 and phase 3 studies has been reported. Treatment exposure of up to 104 weeks with tirzepatide did not increase the risk of a composite outcome of four major adverse cardiovascular events in people with type 2 diabetes across a spectrum of diabetes durations and cardiovascular risk levels.7
Side effects
The adverse events reported in the clinical trials of tirzepatide are very similar to those seen in trials of GLP-1 receptor agonists and mainly involve the gastrointestinal system. For the glucose-lowering indication, these are categorised as:
Very common (affecting one in every 10 people): Nausea, diarrhoea, hypoglycaemia when used with a sulfonylurea or insulin.
Common (affecting one in 10–100 people): Decreased appetite, vomiting, constipation, abdominal pain, dyspepsia, abdominal distention, eructation, flatulence, gastro-oesophageal reflux disease, hypoglycaemia when used with metformin plus an SGLT2 inhibitor, increased heart rate, increased lipase, increased amylase, hypersensitivity reactions, fatigue and injection site reactions.
Information for prescribers
Cautions/contraindications
Hypersensitivity to the active substance or to any of the excipients (sodium phosphate dibasic heptahydrate, sodium chloride, concentrated hydrochloric acid and sodium hydroxide, glycerol, phenol, benzyl alcohol, water).
Prescribing
Tirzepatide is available in six doses, three of which (5 mg, 10 mg and 15 mg once weekly) are maintenance doses. It is currently available in the KwikPen® pre-filled injection device, each pen containing four doses of the specific strength (i.e. one month’s supply). Needles will also need to be prescribed since they are not included with the KwikPen. Detailed instructions for use are available here.
Tirzepatide is injected subcutaneously in the abdomen, thigh or upper arm. Injection sites should be rotated with each dose, and at a different site to insulin if this is also prescribed. It should be injected once weekly on the same day each week and can be administered at any time of day without reference to meals. If a person misses a dose, it can be given within four days; otherwise, the dose should be omitted for that week.
Initiation
Tirzepatide should be initiated at a dose of 2.5 mg weekly and then increased to 5 mg weekly after four weeks. In the SURPASS programme, the majority of the glucose-lowering effect was seen at the 5 mg dose, with only modest improvements in HbA1c thereafter. Since the price of tirzepatide increases as the maintenance dose rises, there may be local pressure to retain patients on the 5 mg dose.
Further increments in dose should be by 2.5 mg (i.e. 5 mg ” 7.5 mg ” 10 mg ” 12.5 mg ” 15 mg weekly) at no less than 4-weekly intervals, so as to reduce the (mainly gastrointestinal) side effects. Based on the SURPASS trials, there is likely to be substantially more weight reduction seen with the higher doses of tirzepatide.
KwikPen recommendations
Eagle-eyed prescribers will have spotted the recommendation for use of a swab during the administration of tirzepatide. This is generic advice for medicines administered via the KwikPen, which I suspect has previously gone under the radar. The Summary of Product Characteristics also highlights the inclusion of benzyl alcohol as a preservative in the KwikPen formulation; thus it is recommended that recipients with renal or hepatic impairment should be warned of the risk of metabolic acidosis. My personal view is that this is a theoretical risk, and it will be low on my list of safety warnings for people in whom I initiate tirzepatide.
Author’s recommendations
Elderly people: There are very limited data from people aged 85 years and over, so I would avoid. Note also the ongoing concerns regarding sarcopenia in the elderly person with type 2 diabetes, which might be worsened by weight loss.
Diabetic retinopathy: I would apply the same restrictions as recommended for GLP-1 RAs (see previous At a glance factsheet)8 – that is, avoid in people with active retinopathy needing ophthalmology review (usually by ensuring that eye screening is up to date).
Hypoglycaemia: Tirzepatide should only cause hypoglycaemia when co-prescribed with insulin and/or sulfonylureas; in such situations, reduction in the doses of these medicines should be considered.
Acute pancreatitis: I would not recommend tirzepatide in people with a history of pancreatitis, and it should be withdrawn and not restarted in patients who have an episode whilst on treatment.
Severe gastrointestinal disease: I would avoid in this scenario (including gastroparesis).
Renal impairment: No dose adjustment is necessary in people with renal impairment, including those with end-stage renal disease. An exploratory analysis and meta-analysis suggest improvement in urinary ACR with tirzepatide, with no worsening of eGFR.9
Liver disease: No dose adjustment is required in people with liver impairment, although I would advise against use in those with severe hepatic impairment, because of the lack of experience in such individuals.
Warfarin and other drugs with a narrow therapeutic index (such as digoxin): The absorption of orally administered medicines can be affected due to slowing of gastric emptying (as with GLP-1 RAs), and so monitoring at initiation of tirzepatide and following dose escalation is recommended.
Oral contraceptives: No dose adjustment of oral contraceptives is required in women with normal BMI; however, there is limited information in women with obesity or overweight. Since reduced efficacy of oral contraceptives cannot be excluded, it is advised to switch to a non-oral contraceptive method or to add a barrier method of contraception when initiating tirzepatide therapy (for 4 weeks), or after each dose escalation (for 4 weeks).
Pregnancy and breastfeeding: Tirzepatide is not recommended during pregnancy and breastfeeding, and the drug should be discontinued at least one month before women attempt to conceive.
Paediatric populations: Tirzepatide is not licensed for use below the age of 18 years.
Contraindications: As per the US licence, I would regard tirzepatide as contraindicated in people with a personal or family history of medullary thyroid carcinoma (MTC) and in those with multiple endocrine neoplasia syndrome type 2 (MEN 2).
Switching from GLP-1 RAs: There have been no clinical trials of switches, and so formal advice from the manufacturer may not be forthcoming. In those on maximal dose of a GLP-1 RA (either subcutaneous or oral), I would initiate tirzepatide at the 5 mg weekly dose. Otherwise, I would start with 2.5 mg weekly but consider reducing the time before uptitration.
Frequently asked questions
Why are the NICE recommendations so different from both tirzepatide’s licensed indications and other international guidelines?
The NICE recommendation was entirely based on the manufacturer’s application for approval. I assume this was made based upon NICE having given identical approvals for the currently recommended GLP-1 RAs, all of which are at odds with international guidelines such as the ADA/EASD Consensus Report.10
Advice on GLP-1 RA use is expected to be updated as part of the latest review of the NICE NG28 guideline on type 2 diabetes management, which has an expected publication date of December 2024.
Is NICE going to consider the weight management licence for tirzepatide?
Yes. NICE is currently appraising “the clinical and cost-effectiveness of tirzepatide within its marketing authorisation for managing obesity or overweight with risk factors”.11 At time of writing, the expected publication date of this Technology Appraisal is the end of May 2024.
There has been a large phase 3 clinical trial programme, SURMOUNT, undertaken in people who do not have diabetes. In these trials, as has been the case with other medicines, the weight reductions observed were much greater than in individuals with diabetes.
How different is tirzepatide from the widely used GLP-1 RAs?
Apart from the greater efficacy for both glucose lowering and weight loss, not very. Although promoted as first in class (which it is), the mode of administration and side-effect profile of tirzeptide are very similar to the well-established weekly subcutaneous GLP-1 RAs, such as semaglutide and dulaglutide. Any diabetes service which currently manages GLP-1 RAs should be able to easily adopt tirzepatide into routine practice.




Small but significant 12% increased risk of developing chronic cough compared to treatment with other second-line agents for type 2 diabetes.
8 Dec 2025