Introduction
Clare Hambling, Chair of PCDS and National Clinical Director for Diabetes and Obesity, NHS England
● Think and manage holistically – consider all aspects of the person with diabetes, obesity and long-term conditions.
● Use the term “Multiple long-term conditions” rather than “Multimorbidity”, as the latter implies poor health which the person cannot influence.1
- Defined as two or more chronic conditions in the same individual, associated with poorer quality of life, greater healthcare needs and increased mortality.
- Manage the conditions collectively.
● Multiple long-term conditions are associated with social deprivation.2
● Globally, diabetes has a high prevalence at all ages, as does diabetes in combination with hypertension, coronary heart disease or other long-term conditions.
● In people with diabetes, between 2003 and 2018, hospitalisation rates for vascular diseases and diabetes have reduced, while rates for cancer, renal disease and infections increased.3
● The 2021 Health Survey for England found that 26% of adults were obese, with a higher proportion of men than women being overweight or obese (69% vs 59%).
- Obesity prevalence was lowest in the least deprived areas and highest in the most deprived (20% vs 34%).
- 11% of obese people had a diabetes diagnosis, compared with 5% of overweight adults and 3% of those not overweight or obese.
● Early-onset type 2 diabetes is now more common than type 1 diabetes in people under 40 years of age.
- Characterised by a more aggressive phenotype with more rapid deterioration, worse cardiometabolic risk profile and higher complications.
- Individuals are less likely to receive all care processes, so additional funding has been made available for extended reviews.4 See How to conduct an extended review for people with early-onset type 2 diabetes.
- Women with early-onset type 2 diabetes and their babies have poorer outcomes; 45% of pregnancies are unplanned, so medication reviews and support to achieve tight glycaemic control are important for women not using effective contraception.
NHS England resources
● 12-week free digital weight management programme:
- For those with BMI ≥30 kg/m2 (≥27.5 kg/m2 for high-risk ethnic groups) and hypertension, diabetes or both.
- Includes triage to three different levels of support according to needs.
- Also available, without referral, for NHS staff (same BMI requirements; valid NHS email address needed).
● Healthier You NHS Diabetes Prevention Programme:
- 20% reduced risk of developing type 2 diabetes in high-risk groups.5
● NHS Type 2 Diabetes Path to Remission Programme:
- Available across England; weight loss from pilots similar to clinical trials.6
● Structured education programmes:
Obesity and diabetes
Matt Capehorn, GPwSI obesity and diabetes and Clinical Manager, Rotherham Institute for Obesity
Pam Brown, GP, Swansea
● In the global IntroDia study, physicians believed medication and behavioural change each contribute around 50% to success in those newly diagnosed with type 2 diabetes. However, is equal balance really given to the two in consultations in the real world?
● Fat cells or adipocytes, particularly those in visceral fat, are not just inert fat storage – they are highly metabolically active cells which contribute to hypertension, inflammation, thrombosis, dyslipidaemia, type 2 diabetes and cardiovascular disease risk.7
- Liposuction removes only the (less dangerous) subcutaneous fat and, by removing some of the fat storage areas, may in fact increase the amount of (more dangerous) visceral fat.
● Total meal replacement for 12–20 weeks followed by dietitian-guided food reintroduction is effective in achieving type 2 diabetes remission – 46% of participants maintained remission at 1 year and 36% at 2 years in the DiRECT study.8
● Behavioural weight management programmes tend to have peak weight loss at 6 months and then some regain; however, weight remains below the ongoing increased weight trajectory of those not offered programmes. Higher attendance generally translates into greater weight loss.9
- Following weight loss, weight regain occurs at around the same speed whether the weight was lost rapidly or gradually; therefore, rapid weight loss may be preferable.10
● Homeostatic adaptation after weight loss results in weight regain – physiological changes over which we have no control result in weight returning close to the individual’s “set point”. Some treatments such as bariatric surgery may help reset the set point.
● The role of psychological hunger and its impact on amount of food consumed varies – encourage people to think about when their desire to eat is likely to be psychologically driven.
● Obesity is a disease of dysregulation of the body’s ability to manage physiological hunger, and a chronic relapsing condition that poses a risk to health; therefore, we should feel comfortable raising weight as often as possible.
● Dr Capehorn challenged the audience to consider whether it was most appropriate to try to prevent obesity by managing the 100+ causes,11 or instead to focus on managing overweight/obesity, highlighting that the strongest evidence is for treatment options.
Diabetes, obesity and chronic kidney disease
Andrew Frankel, Consultant Renal Physician, Imperial College Healthcare NHS Trust
Waqas Tahir, GPwER Diabetes, Bradford
● Earlier age of type 2 diabetes diagnosis and longer survival due to good management will result in significantly greater numbers of people with chronic kidney disease (CKD) and need for renal replacement therapy. Obesity also increases CKD risk. We have the ability to prevent this.
● Five key tasks:
- Recognise – requires both eGFR and ACR; diagnose early.
- Code – so that we can find people and check we are managing well.
- Treat – ACE inhibitor/ARB; SGLT2 inhibitor; blood pressure optimisation; finerenone if criteria met and potassium ≤4.8 mmol/L; glycaemic control (care with hypoglycaemia risk if CKD).
- Reduce cardiovascular risk – lipid optimisation; lifestyle, including smoking cessation.
- Engage the person with CKD – use the term “kidney health check”.
● Optimise ACE inhibitor or ARB rapidly unless frail or eGFR <25 – consider consulting renal colleagues.
● If heart failure and on spironolactone, do not switch to finerenone at present.
● If already significant renal damage (eGFR <60), optimising treatment could delay need for dialysis by 13 years.13
● Prioritise treating as many people as possible to reduce the long-term burden – automate as much as possible and find a sequence for adding drug therapies that suits the person with CKD.
● Three top tips:
- Use the term “kidney health check” to encourage both eGFR and ACR testing.
- Recognise that the earlier you start to manage, the more effective the treatment.
- Look for those who are not attending and invite them in for testing.
● Safe prescribing of SGLT2 inhibitors video – https://medicalcare.rcp.ac.uk/content-items/video/medication-safety-shorts/
Diabetes, obesity and cardiovascular disease
Ameet Bakhai, Consultant Cardiologist and Cardiovascular R&D Director, Royal Free London NHS Trust
Naresh Kanumilli, GPwSI Diabetes and Cardiology, Manchester
● Clinical inertia from clinicians and people with diabetes kills – the evidence base has long been clear on how to prevent and manage cardiovascular disease (CVD) and diabetes. Clinicians need to take action urgently to change from reactive to proactive healthcare.
● Results within the so-called normal range may already be demonstrating increased risk (e.g. hsCRP or BNP/NT-ProBNP).
● Even if blood pressure appears well controlled, if there is loss of night-time dipping then this is a sign of endothelial damage.
● View and be guided by the 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes.12
● Lifestyle is very important – weight reduction, structured exercise training, increased physical activity, wearable tracker devices, smoking cessation, Mediterranean diet.
● Use SCORE2-Diabetes risk score from the ESC guidelines to identify 10-year CVD risk in those without symptomatic atherosclerotic CVD or severe target end-organ damage (Figure 1).
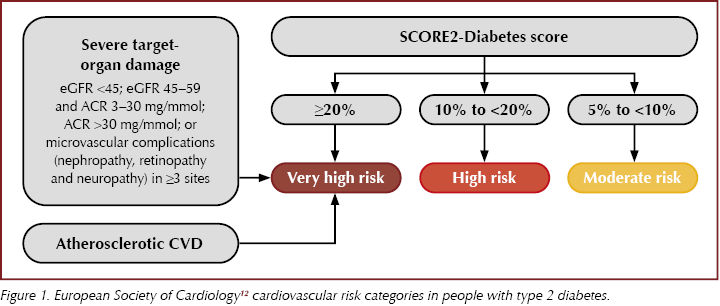
● Those with established or very high risk of atherosclerotic CVD should all receive an SGLT2 inhibitor or GLP-1 receptor agonist, as well as metformin if glycaemic control not achieved, and consider pioglitazone for further glucose lowering if no heart failure.
● LDL reduction is important to prevent cardiovascular events – use ESC targets and consider additional non-HDL targets:
- Very high risk: LDL <1.4 mmol/L (non-HDL <2.2 mmol/L).
- High risk: LDL <1.8 mmol/L (non-HDL <2.6 mmol/L).
- Moderate risk: LDL <2.6 mmol/L.
● Use high-potency statins (atorvastatin, rosuvastatin), +/– ezetimibe and bempedoic acid (can be used as a combined preparation), and consider icosapent ethyl for elevated triglycerides, and inclisiran in primary care within NICE recommendations.
● 6 weeks’ reduction in lipids with a statin makes a mortality difference after around 2 years, while an SGLT2 inhibitor can reduce mortality as early as 4 months from initiation.
● Remember that conditions such as metabolic dysfunction-associated liver disease (MASLD, previously termed NAFLD) and obstructive sleep apnoea can increase CVD risk by 60% each, so identify these people and optimise CVD risk factors.
Diabetes, obesity and cancer
Safwaan Adam, Consultant Endocrinologist, The Christie NHS Foundation Trust
Hannah Beba, Consultant Pharmacist, Leeds Health and Care Partnership
● 6–7% of the general population have diabetes but 20% of people with cancer have diabetes.
● Childhood cancer survivors have a 39% increased relative risk of diabetes. If abdominal radiation, more likely to develop type 1 diabetes as adult.
● Higher HbA1c is associated with increased cancer risk in some studies.
● New onset of type 2 diabetes or a rapid increase in HbA1c in pre-existing type 2 diabetes may be presenting signs of pancreatic cancer.
● Commonly used diabetes drugs:
- Metformin may decrease cancer risk.
- Pioglitazone may increase bladder cancer risk.
- Insulin may increase cancer risk.
- Other drugs are probably neutral; recent paper associating GLP-1 receptor agonists with thyroid cancer was of poor quality.
● JBDS-IP diabetes and cancer guideline has useful and important guidance and algorithms.14
● Immune checkpoint inhibitors may rarely cause a sudden onset of type 1 diabetes, which can develop over a very short period and presents with diabetic ketoacidosis in 50–75% of cases.
- This is not reversible and, although rare, is high-risk for missed diagnosis.
- People treated with these drugs and their clinicians should be aware of this risk.
- Further information on immune checkpoint inhibitors and diabetes is open access at: https://doi.org/10.2337/dc23-1964.15
● One in two people treated with dexamethasone at doses used in cancer therapy get significant hyperglycaemia; dexamethasone is used for a variety of purposes in oncology.
● JBDS-IP management of hyperglycaemia with steroid therapy guideline contains useful algorithms for monitoring and management.16
● If childhood cancer survivors develop hyperphagia, consider hypothalamic obesity and specific treatment available.
● It is important to manage diabetes in people with cancer as this can improve their symptoms, quality of life and response to treatment.






Risk ratios of 1.25 for autism spectrum disorder and 1.30 for ADHD observed in offspring of mothers with diabetes in pregnancy.
18 Jun 2025