Case presentation
Simon, a 76-year-old man with a two-year history of type 2 diabetes, sought a private endocrinology consultation because of ongoing symptoms of weight loss and fatigue. He also reported loss of appetite but denied polyuria and polydipsia. There had been no significant abdominal pain and no change in bowel habit.
Metformin had been commenced shortly after the diagnosis of diabetes but was discontinued because of gastrointestinal intolerance. Dapagliflozin had been tried but was stopped because of continued weight loss. Simon was now taking alogliptin but good glycaemic control remained evasive.
Past medical history: Hypertension; dyslipidaemia; peripheral vascular disease.
Medication: Amlodipine 5 mg once daily; atorvastatin 20 mg once daily; clopidogrel 75 mg once daily; alogliptin 25 mg once daily.
Social history: Retired; non-smoker; alcohol 9 units/week.
Family history: One brother with type 1 diabetes; one brother with diet-controlled type 2 diabetes; one brother with pancreatic cancer.
Examination: Blood pressure 128/67 mmHg; BMI 19.4 kg/m2. Cardiovascular and respiratory systems unremarkable. Abdomen – palpable liver edge, no other masses.
What is your clinical assessment of the situation? What further investigations would you consider?
New-onset diabetes in a thin older adult
It is important to consider different underlying types of diabetes in every new diagnosis of diabetes, a point well illustrated in the case of a non-overweight/obese elderly person (ElSayed et al, 2023). Box 1 lists the possible diagnoses for an older person, noting that maturity-onset diabetes of the young (MODY) should have manifested itself at a younger age and that gestational diabetes would be restricted to pregnancy. Table 1 summarises some clinical features and investigations that can help differentiate between these possibilities (Morris, 2020).

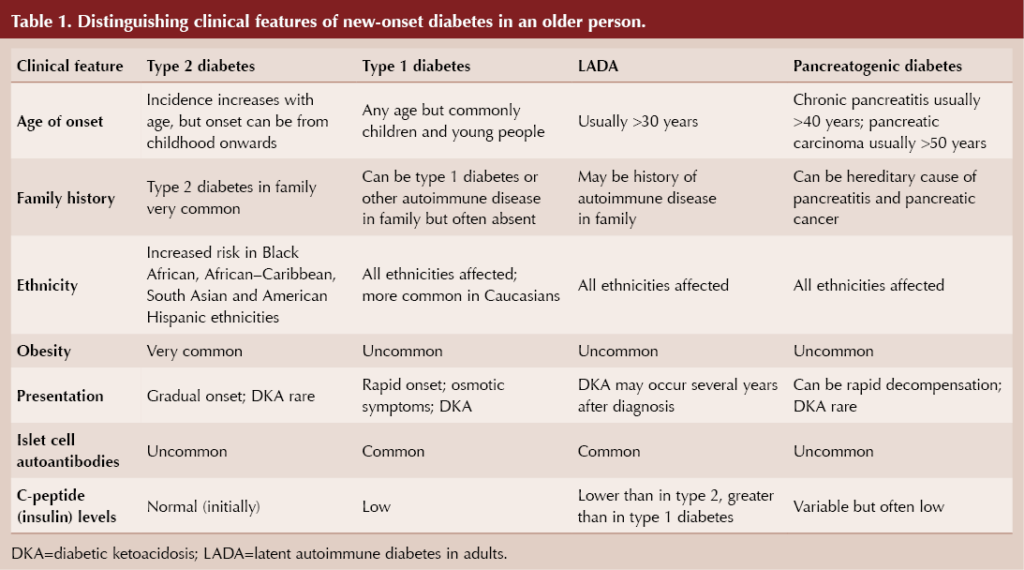
Classical symptoms of diabetes, including thirst, polyuria, weight loss, fatigue and recurrent infection, may be present in any type of diabetes but are likely to be more pronounced with type 1 diabetes. With rising levels of obesity, it is inevitable that a greater proportion of those with types of diabetes other than type 2 will be overweight or obese at the time of diagnosis.
Latent autoimmune diabetes in adults (LADA) is, like type 1 diabetes, an autoimmune condition with antibodies directed against pancreatic beta-cells. Typically, individuals with LADA are not obese and can initially be managed with oral hypoglycaemic agents, so they are frequently diagnosed as having type 2 diabetes. Subsequently, however, they progress, often over several years, to an absolute requirement of insulin, by which time decompensation to diabetic ketoacidosis is a risk (Naik and Palmer, 2003; Buzzetti et al, 2020).
Possible causes of pancreatogenic diabetes, also known as type 3c diabetes, are listed in Box 2, with chronic pancreatitis accounting for around 75% of cases (Duggan and Conlon, 2017; Gupte et al, 2018). Exocrine (digestive enzyme production) as well as endocrine (hormone-secreting) functions of the pancreas can be affected in pancreatogenic diabetes, so it is important to ask about gastrointestinal symptoms: upper abdominal pain, steatorrhoea, bloating and weight loss. Additional symptoms of jaundice, anorexia and unexpected deterioration in glycaemic control would strengthen concern over the likelihood of pancreatic carcinoma (Cui and Anderson, 2012). Alcohol and smoking history are particularly relevant. Again, pancreatogenic diabetes is frequently misclassified, often as type 2 diabetes.

Case analysis
Simon developed new-onset diabetes at the age of 74 years. His BMI was not raised and he had continued to lose weight since diagnosis. Glycaemic control had deteriorated since the time of diagnosis despite oral medication for type 2 diabetes. This situation is suggestive of insulin deficiency rather than insulin resistance.
The original diagnosis of type 2 diabetes needs to be questioned. LADA is a possible diagnosis given the clinical picture and in light of the family history of autoimmune diabetes. Although there is no history to indicate pancreatitis, pancreatogenic diabetes should also be considered; in particular, Simon’s age, BMI, symptoms and family history raise the possibility of pancreatic cancer as a cause of his diabetes.
Case outcome
A full set of routine blood tests was requested, along with C-peptide levels to assess insulin reserve. Also requested were an islet cell autoantibody test, which would support a diagnosis of LADA, and a CA 19-9 level which, if raised, would support a diagnosis of pancreatic carcinoma, although it is not specific to this condition. A CT scan and upper gastrointestinal endoscopy were also arranged. Simon was given a meter and test strips to monitor his glucose and ketone levels.
Investigations
FBC, U+E, LFTs and calcium: normal range.
HbA1c: 81 mmol/mol (9.6%).
C-peptide level: Low.
Islet cell autoantibodies (anti-GAD, IA2 and Zn transporter antibodies): Negative.
CA 19-9 level: 167 U/mL (normal range=0–37 U/mL).
Urgent CT scan abdomen: Pancreatic carcinoma with multiple hepatic lesions.
Urgent upper gastrointestinal endoscopy: No abnormality detected.
Fingerprick glucose levels: Pre-breakfast 7–11 mmol/L; pre-lunch 14–20 mmol/L; pre-evening meal 13–18 mmol/L.
Further management
● Gliclazide commenced at 40 mg once daily with breakfast, with gradual uptitration to 240 mg at breakfast and 80 mg at evening meal, with continued glucose monitoring.
● Gastroenterology: Pancreatic enzyme supplements with meals.
● Endoscopic ultrasound and biopsy of pancreas: Neuroendocrine carcinoma of pancreas.
● Freestyle Libre glucose monitoring instituted: High daytime glucose readings peaking at evening meal, with levels dropping to 4–8 mmol/L overnight.
● Humulin I, an intermediate-acting basal insulin, was subsequently commenced at 4 units with breakfast, with uptitration every 3 days according to pre-evening glucose readings, aiming to control high daytime levels and avoid risk of nocturnal hypoglycaemia.
● Alogliptin was discontinued.
● Referral to oncology: Palliative chemotherapy was arranged.
Pancreatic cancer
Symptoms of pancreatic cancer (see Box 3) often present late, and most are non-specific so they may be misinterpreted as dyspepsia, gallstone disease or irritable bowel syndrome (Bennett, 2018). As a consequence, the cancer may be well advanced and not amenable to surgical treatment by the time it is diagnosed. At the point of diagnosis, 80–85% of cases of pancreatic ductal adenocarcinoma are found to be locally advanced or to have distant metastases (Singhi et al, 2019).

The prognosis for pancreatic cancer is very poor, with a 5-year survival rate of 7.3%, although this improves to 30% for early-stage diagnosis (Pancreatic Cancer Action, 2023). Individuals diagnosed with stage 1 disease survive longer than those diagnosed with more advanced-stage pancreatic cancer (Poruk et al, 2013). Alongside late-stage diagnosis, a high degree of tumour resistance to chemotherapy compounds poor outcomes.
It should be emphasised that pancreatic cancer is a rare condition, with an incidence of around 10 per 100 000 population per year (Sharp et al, 2021).
Pancreatic cancer and diabetes
The relationship between pancreatic cancer and diabetes appears to be bidirectional (Pereira et al, 2020). Thus, people with long-standing diabetes are at increased risk of pancreatic cancer, whilst those with recently diagnosed type 2 diabetes have a 5-fold increased relative risk of pancreatic periductal adenocarcinoma (the most common form of pancreatic cancer) compared with the general population (Ben et al, 2011).
Prediabetes or diabetes is found in around 80% of people diagnosed with pancreatic carcinoma and, importantly, is often identified a year or two ahead of this diagnosis (Wang et al, 2003). A retrospective study found that blood glucose levels began to rise 30–36 months ahead of the diagnosis of periductal adenocarcinoma (Sharma et al, 2018).
Whilst beta-cell destruction may be one factor that contributes to the development of diabetes in people with pancreatic cancer, other mechanisms are also at play. Hyperglycaemia ensues even when the tumour size is small with little pancreatic destruction, and normoglycaemia can be restored after cancer resection (Singhi et al, 2019; Sharp et al, 2021). Insulin resistance accompanies pancreatic cancer, and local hormonal (paracrine) effects appear to be in operation (Hart et al, 2016).
New-onset diabetes, then, represents an opportunity for early detection of pancreatic carcinoma. Up to 0.9% of individuals aged over 50 years with new-onset diabetes have diabetes secondary to pancreatic ductal adenocarcinoma (Singhi et al, 2019).
When to suspect pancreatic cancer in the person with diabetes
Consider the possibility of pancreatic cancer in new-onset diabetes in an individual with normal or low BMI. This rare possibility is more likely with increasing age, notably in people over 50 years (Pereira et al, 2020), and if other risk factors for pancreatic cancer – smoking, alcohol, chronic pancreatitis and family history of the condition – are present. Clearly, if these factors are accompanied by symptoms consistent with pancreatic cancer (see Box 3), the risk of pancreatic cancer being present is enhanced.
For those with pre-existing diabetes, a sudden deterioration in glycaemic control not accounted for by lifestyle or medication change could signal the need to investigate for pancreatic cancer (Mueller et al, 2018; Sharp et al, 2021).
As pancreatic cancer is a rare condition, unfiltered screening of the asymptomatic population does not take place, but there is a strong case for screening groups at high risk of the condition, including those with new-onset diabetes (Pereira et al, 2020). It has been suggested that primary care could undertake a regular audit to identify individuals with a new diagnosis of type 2 diabetes and a normal or low BMI, who could then be screened for pancreatic cancer. In a GP practice of 10 000 people, this would typically be up to five people per year (Bennett, 2018).
What action to take
The NICE (2023) recommendations on recognition and referral of suspected pancreatic cancer are summarised in Box 4. Notably, the guideline states that imaging should be considered for individuals aged 60 years or over with new-onset diabetes (NICE, 2023).

If pancreatic carcinoma is suspected, the key investigation to request is an urgent CT scan of the abdomen, which has sensitivity and specificity approaching 100%. An ultrasound scan is a less sensitive alternative, such that it is possible for a person with new-onset diabetes and no worrying symptoms to have a normal ultrasound and yet still have pancreatic cancer (Bennett, 2018). An MRI scan may be undertaken later to provide further detail.
Unfortunately, there are no validated biomarkers for early detection of pancreatic cancer. A raised CA 19-9 level would be expected; however, this is not very specific for the condition (Pereira et al, 2020). Routine blood tests should be undertaken, although normal results do not exclude the diagnosis – in the present case report, the individual with metastatic pancreatic cancer was not anaemic and his LFTs were undisturbed.
The possibility of autoimmune diabetes can be investigated by performing a pancreatic autoantibody screen. Insulin reserve can be assessed by measuring C-peptide level, which can then predict the need for insulin therapy.
Article learning points
● Many of the symptoms of pancreatic cancer are non-specific, so diagnosis is often made at a late stage, with correspondingly poor prognosis.
● Earlier diagnosis of pancreatic cancer could improve survival rates.
● The majority of people with pancreatic cancer have prediabetes or diabetes.
● Consider the possibility of pancreatic cancer in people over the age of 50 years with new-onset diabetes who have a normal or low BMI, and also in those with pre-existing diabetes who experience a sudden unexplained deterioration in glycaemic control or excessive weight loss.
● Pancreatic autoantibody testing in people with new-onset diabetes who have a normal or low BMI may help identify those with late-onset type 1 diabetes or LADA.
● A CT scan is the key investigation for suspected pancreatic cancer.




Small increases in three lifestyle behaviours is effective in reducing all-cause mortality.
27 May 2025