Primary prevention: Assessing cardiovascular risk
QRISK3 (available at: https://qrisk.org/index.php) is generally the tool of choice for cardiovascular disease (CVD) risk assessment in the UK. However, it is important to note that this should not be used in the following groups:1
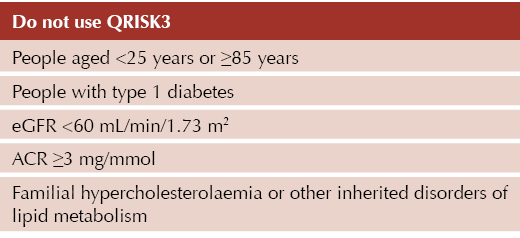
For people with type 2 diabetes, regardless of other risk factors, males aged ≥52 years and females aged ≥60 years will generate a QRISK score of over 10%, thus qualifying them for primary prevention of cardiovascular disease and treatment according to NICE CG181.1 Therefore, a large proportion of people living with type 2 diabetes are potentially eligible for lipid-lowering therapy.
Note that QRISK3 is only valid for patients who do not already have a diagnosis of coronary heart disease (including angina or heart attack) or stroke/transient ischaemic attack. CVD risk tools may also underestimate risk in certain groups of people (e.g. HIV, taking medications to treat CVD risk factors, recently stopped smoking, taking medicines that can cause dyslipidaemia such as immunosuppressant drugs, severe mental illness, autoimmune disorders, other systemic inflammatory disorders).1
Statins
Statins remain the preferred first-line treatment for primary prevention of CVD. For people with a QRISK3 score of ≥10%, atorvastatin 20 mg should be offered (making use of the NICE patient decision aid, if helpful), which can then be titrated as needed.
Where a person at high cardiovascular risk reports potential intolerance to recommended high-intensity statin treatment, the NHS Accelerated Access Collaborative’s Statin Intolerance Pathway can be referred to.
If lipid targets are not achieved on the maximally tolerated statin dose, combination lipid-lowering therapy should be considered. A useful summary of NHS and NICE guidance on lipid management is available here.
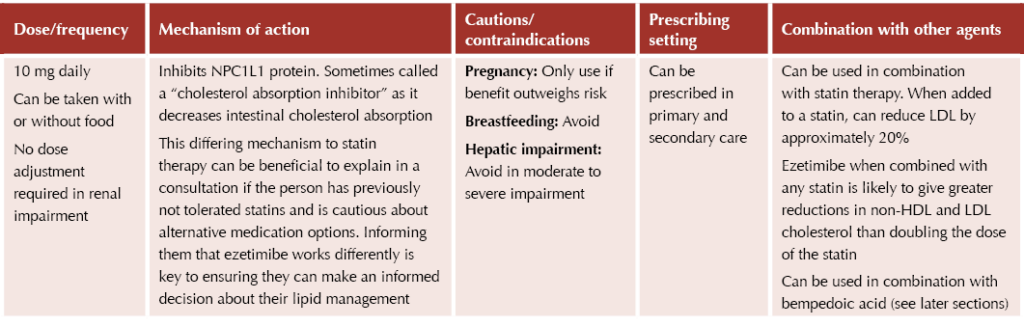
Ezetimibe
Ezetimibe (Ezetrol®) provides an option for those who do not achieve treatment targets (a >40% reduction in non-HDL cholesterol)1 in the following scenarios:2
- After 3 months of maximal statin therapy.
- If statin treatment is contraindicated or not tolerated.
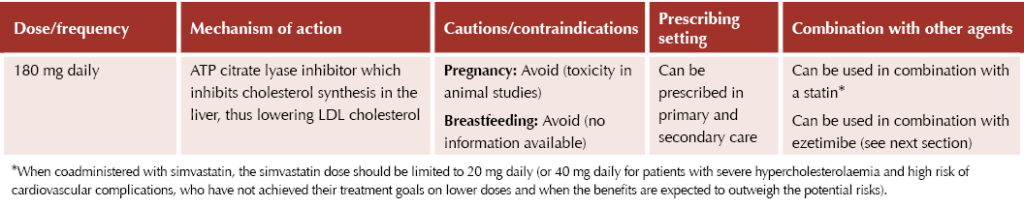
Bempedoic acid
Bempedoic acid (Nilemdo®) is a relatively new therapy that works similarly to statins; thus, it can be considered a good option for true statin-intolerant individuals. It is licensed for use in combination with a statin ± other lipid-lowering therapies, or with other lipid-lowering therapies or alone if a statin is contraindicated or not tolerated, for people who have not responded adequately to other appropriate measures.
The agent has recently been shown to reduce the risk of major adverse cardiovascular events (MACE) by 13% in people with established or high risk of CVD.3
Bempedoic acid/Ezetimibe combination
As per NICE TA694 guidance,4 bempedoic acid with ezetimibe is an option when statins are contraindicated or not tolerated and when ezetimibe alone does not control LDL cholesterol sufficiently. Bempedoic acid when combined with ezetimibe produces an additional LDL cholesterol reduction of approximately 28% (range 22–33%), but no clinical outcome evidence for this specific combination is currently available.
Treatment can be with separate tablets (Nilemdo®) or a fixed-dose combination (Nustendi®), which reduces tablet burden for patients. Nustendi® contains 180 mg bempedoic acid and 10 mg ezetimibe, enabling once-daily dosing.
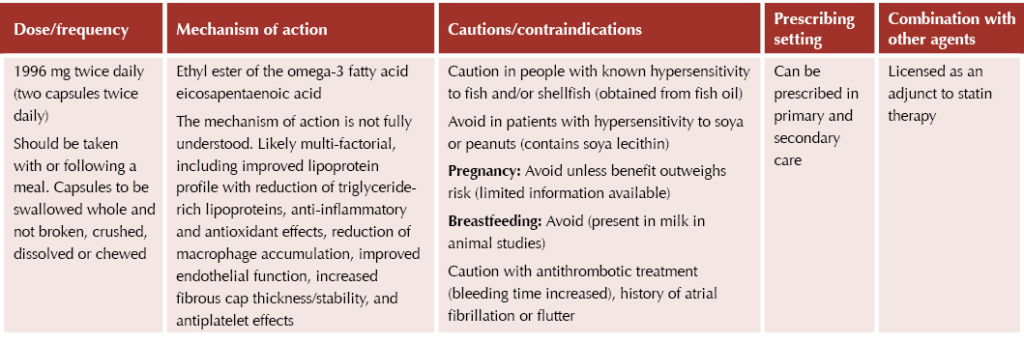
Icosapent ethyl
Granted NICE approval in July 2022, icosapent ethyl (Vazkepa®) is the newest lipid-lowering therapy available in the UK market. NICE TA805 recommends icosapent ethyl as an option for secondary prevention of cardiovascular events in adults.5 It is recommended if the person has a high risk of cardiovascular events and raised fasting triglycerides (≥1.7 mmol/L) and is taking statins, but only if they have:
● Established CVD (defined as a history of acute coronary syndrome, coronary or other arterial revascularisation procedures, coronary heart disease, ischaemic stroke or peripheral arterial disease),
and
● LDL cholesterol levels above 1.04 mmol/L and ≤2.60 mmol/L.
These recommendations were made as there are currently no treatment options to reduce the risk of cardiovascular events in people taking statins who have controlled levels of LDL cholesterol but raised levels of triglycerides. Thus, this agent is suitable for a very specific cohort of patients. It was previously shown to reduce four-point MACE risk by 26% in such a cohort.6
PCSK9 inhibitors
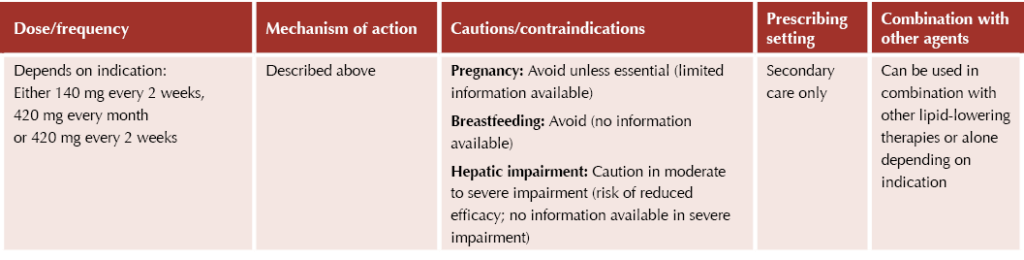
The PCSK9 inhibitors alirocumab (Praluent®) and evolocumab (Repatha®) have tight criteria for use and are restricted to secondary care prescribing only. They are potent medicines which can result in large reductions in lipid levels. They are both subcutaneous injectable agents which can be self-administered by the patient at home.
NICE criteria for use of PCSK9 inhibitors are summarised in the table below:7,8
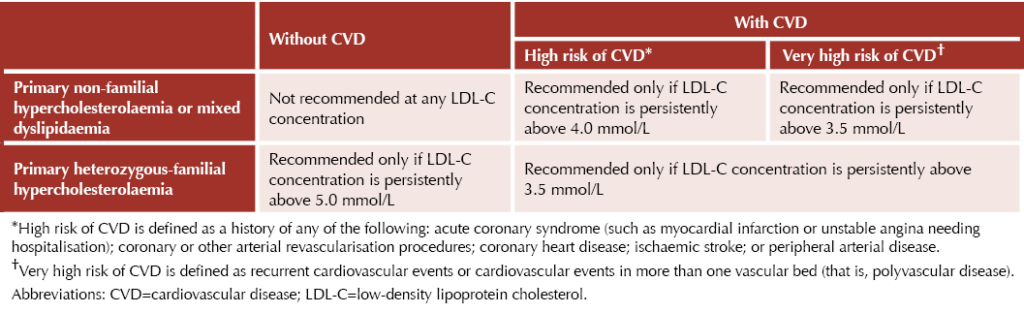
Alirocumab (Praluent®)
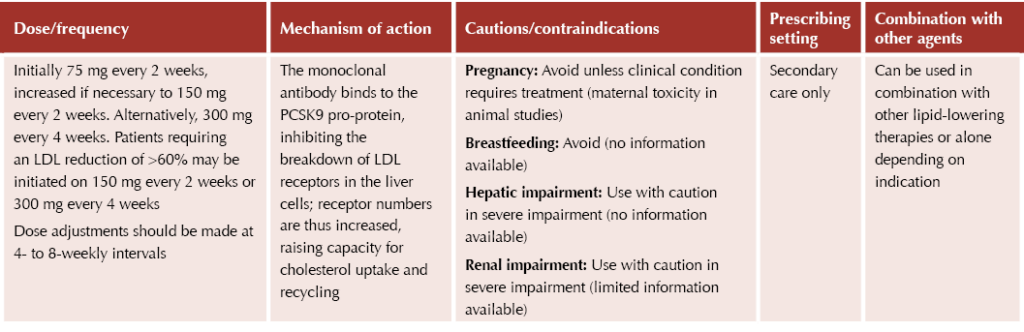
Evolocumab (Repatha®)
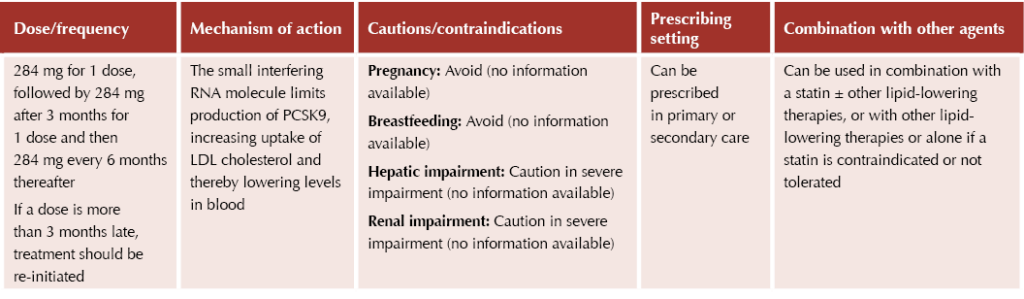
Inclisiran
Inclisiran (Leqvio®) is another new drug for lipid management that acts via the PCSK9 protein, in this case by inhibiting its production. As a newer agent, there is limited data on outcomes for inclisiran at present. Inclisiran can be prescribed in primary or secondary care, although roll-out in primary care has been met with concern from the RCGP and BMA.9
Inclisiran is an injectable agent, and this is something to be discussed with patients when considering initiation; some may benefit and prefer the reduced frequency of the injectable route, while others may prefer oral options listed previously.
NICE TA733 recommends inclisiran as an option for treating primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia as an adjunct to diet in adults. It is recommended only if:10
● There is a history of acute coronary syndrome (e.g. myocardial infarction or unstable angina needing hospitalisation), coronary or other arterial revascularisation procedures, coronary heart disease, ischaemic stroke or peripheral arterial disease,
and
● LDL cholesterol concentrations are persistently ≥2.6 mmol/L despite maximum tolerated lipid-lowering therapy (i.e. maximum tolerated statins with or without other lipid-lowering therapies, or other lipid-lowering therapies when statins are not tolerated or are contraindicated).
Summary
Lipid management remains an intrinsic part of management for people living with type 2 diabetes to reduce their cardiovascular risk. The range of therapies available has expanded in recent years; however, each has different criteria for use and so should be checked on each occasion prior to prescribing to ensure the person meets the appropriate indications for use.
Reassessment of cardiovascular risk and review of whether lipid-lowering therapy is needed should be done opportunistically in the diabetes review, remembering that checking cholesterol is one of the eight annual care processes for people living with diabetes.
Individualised discussion with the person living with diabetes is imperative to ensure that an informed decision is made on treatment and the treatment choice.




Jane Diggle discusses emotional health and diabetes distress, and offers some tips for discussing this in our consultations.
11 Nov 2025