Towards the end of 2017, SIGN (the Scottish Intercollegiate Guidelines Network) published updated guidance on the pharmacological management of glycaemic control in people with type 2 diabetes. SIGN 154 (2017) introduces some key new clinical recommendations and departs significantly from the NICE guideline on the management of type 2 diabetes in adults (NICE, 2015).
In the previous issue of the Journal, I outlined the main recommendations from SIGN 154 for primary care clinicians caring for people with type 2 diabetes (Fernando, 2018). This article provides a fuller analysis of the pharmacological aspects of the guideline and the evidence that underpins its recommendations. It also reproduces the guideline’s practical algorithm for glucose lowering in type 2 diabetes (Figure 1).
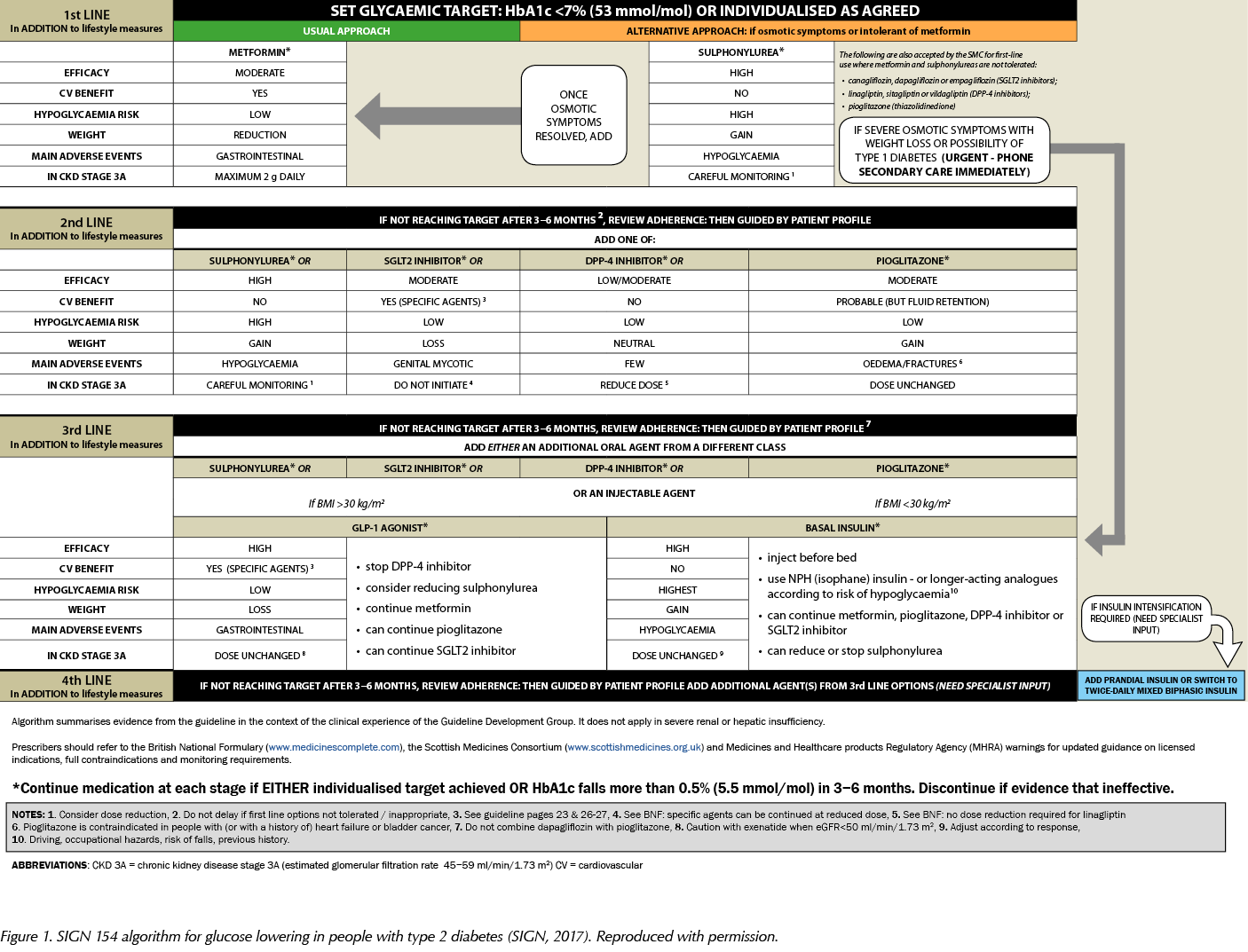

Pharmacological management of glycaemic control in people with type 2 diabetes
Metformin
Metformin remains first-line pharmacological therapy for those with type 2 diabetes. Metformin is moderately potent, does not cause weight gain and, overall, has a low risk of hypoglycaemia. Notably, it has proven cardiovascular (CV) benefit from the seminal UK Prospective Diabetes Study (UKPDS) (1998). Specifically, those individuals on metformin in the UKPDS were demonstrated to have improvements in diabetes-related outcomes and diabetes-related death as well as all-cause mortality. They also had a significantly reduced risk of myocardial infarction (MI).
The use of metformin is limited by its gastrointestinal side-effects, and the axiom “start low, go slow” when prescribing metformin is useful to mitigate these adverse effects. Metformin should also be prescribed with caution in those with moderate renal impairment to avoid the putative risk of lactic acidosis. However, a high-quality Cochrane review (Salpeter et al, 2006) found no significant increased risk of lactic acidosis with metformin from 59321 patient-years of use. It appears to be associated heart failure, liver failure or renal impairment that predisposes to lactic acidosis, rather than metformin per se. SIGN 154 directs us towards the British National Formulary (BNF) and Summary of Product Characteristics (SPC) for metformin to correctly dose metformin at the different stages of renal impairment.
Sulfonylureas
Sulfonylureas (SUs) are cornerstones of all current diabetes guidelines, and SIGN 154 reaffirms their position as first-line oral agents in those who are intolerant of, or who have contraindications to, metformin. SUs are potent glucose-lowering drugs and can be useful in those presenting with marked osmotic symptoms, such as thirst or polyuria. SIGN 154 reminds us that if osmotic symptoms are severe or if there is a history of weight loss, or if type 1 diabetes is suspected, immediate specialist advice should be sought.
The use of SUs is limited by weight gain and, overall, a high risk of hypoglycaemia. Weight gain varies between around 1.5 and 2.5 kg compared to placebo (Nichols and Gomez-Caminero, 2006). The UK Hypoglycaemia Study (2007) demonstrated that the frequency of hypoglycaemia in those treated with SUs was similar to the hypoglycaemia rate during early insulin use in type 2 diabetes. Furthermore, one in 10 of those with type 2 diabetes taking SUs suffered a major hypoglycaemic event each year. Consequently, SIGN 154 recommends prescribing SUs with caution in those who are vulnerable, such as older people, and also in the context of mild-to-moderate renal impairment. SUs are excreted via the kidney and chronic kidney disease (CKD) is an independent risk factor for hypoglycaemia. Therefore, the combination of an SU and CKD is a potent harbinger of hypoglycaemia. SIGN 154 once again directs us towards the BNF and relevant SPCs for SUs to guide safe dosing in renal impairment.
Thiazolidinediones
SIGN 154 suggests we consider pioglitazone in dual or triple therapy in those with type 2 diabetes. Pioglitazone is a moderately potent glucose-lowering agent and, aside from metformin, is the only other oral treatment option that directly reduces insulin resistance, which is one of the key pathophysiological abnormalities in those with type 2 diabetes. Pioglitazone also has probable CV benefit; subgroup analyses from the PROactive trial demonstrated a reduction in non-fatal and fatal MI and recurrent stroke (Wilcox et al, 2007).
SIGN 154 reminds us that pioglitazone is associated with a number of adverse effects. Pioglitazone tends to cause weight gain; expected gain when it is added to insulin varies from 3–4 kg, depending on the dose of pioglitazone. Pioglitazone causes both peripheral and central fluid retention, and therefore should not be used in heart failure and should be prescribed with caution in those with macular oedema. There is also an increased risk of fracture with pioglitazone in both women and men in a dose-dependent fashion (Colhoun et al, 2012). Absolute risks remain small; however, it would be prudent to assess fracture risk if considering pioglitazone and avoid it in those at high fracture risk.
The Medicines and Healthcare products Regulatory Agency (MHRA) has warned about an association between pioglitazone and bladder cancer (MHRA, 2011), and advised not to use pioglitazone in those with a history of bladder cancer or in those with uninvestigated haematuria. This was echoed in SIGN 154. However, the US Food and Drug Administration-mandated Kaiser Permanente Northern California safety study (Lewis et al, 2015), which involved nearly 200000 patients, found no compelling association between pioglitazone and the risk of bladder cancer. However, the MHRA advice above still stands and we should consider the individual risk–benefit ratio when prescribing pioglitazone.
SIGN 154 endorses the use of pioglitazone at CKD stage 3A; helpfully, it can also be used in end-stage renal disease (ESRD), with no dose titration required. It should not be used in hepatic impairment. According to the BNF, pioglitazone is rarely associated with liver dysfunction; however, we have recent evidence that long-term pioglitazone treatment is safe and effective in people with prediabetes or type 2 diabetes and non-alcoholic steatohepatitis (Cusi et al, 2016).
Dipeptidyl peptidase-4 inhibitors
SIGN 154 suggests we consider dipeptidyl peptidase-4 (DPP-4) inhibitors (gliptins) in dual or triple therapy for lowering HbA1c in those with type 2 diabetes. Gliptins are low to moderately potent glucose-lowering agents, are weight-neutral and, overall, have a low risk of hypoglycaemia. Gliptins are generally well tolerated, with few significant side-effects.
Gliptins have no proven CV benefit, but have an established CV safety profile from three high-quality CV outcome trials (CVOTs): SAVOR-TIMI 53 (saxagliptin; Scirica et al, 2013), EXAMINE (alogliptin; White et al, 2013) and TECOS (sitagliptin; Green et al, 2015). Notably, these three trials did not observe an increased risk of pancreatitis with gliptins, which had previously been highlighted as a concern by the MHRA (2012).
Gliptins can be used at all stages of renal impairment, albeit at reduced doses. Uniquely, linagliptin does not require dose titration in renal impairment as it is excreted in the bile. SIGN 154 once again directs us towards the BNF and relevant SPCs for correct dosing of gliptins in the context of renal impairment.
Sodium–glucose cotransporter 2 inhibitors
SIGN 154 recommends that we should consider SGLT2 inhibitors as add-on therapy to metformin in those with type 2 diabetes.
SGLT2 inhibitors are moderately potent glucose-lowering drugs, can lead to weight loss and, overall, have a low risk of hypoglycaemia. Consistent with current licensing, all currently available SGLT2 inhibitors can only be initiated if estimated glomerular filtration rate (eGFR) is >60 mL/min/1.73 m2. If eGFR subsequently falls below 60 mL/min/1.73 m2, dapagliflozin should be stopped, and only lower doses are recommended for empagliflozin and canagliflozin. Further details can be found in the BNF and relevant SPCs.
Importantly, empagliflozin and canagliflozin have proven CV benefit from the landmark EMPA-REG OUTCOME (empagliflozin) study (Zinman et al, 2015) and CANVAS (canagliflozin) trial programme (Neal et al, 2017). Empagliflozin demonstrated significant reductions in CV death, all-cause death and hospitalisation for heart failure compared with placebo in a high CV risk population. Similarly, canagliflozin demonstrated a significant reduction in a primary composite CV endpoint as well as a reduction in hospitalisation for heart failure, again in a high CV risk population. Both drugs also demonstrated a small, but statistically significant, reduction in the progression of renal disease.
Propelled by these pivotal studies, SIGN 154 recommends that SGLT2 inhibitors with proven CV benefit (currently only empagliflozin and canagliflozin) should be preferentially considered in those with type 2 diabetes and established CV disease.
Adverse effects of SGLT2 inhibitors include genital mycotic infections and, to a lesser extent, urinary tract infections. Osmotic symptoms, such as thirst and polyuria, may also be encountered. Hypotension and dizziness may also occur owing to intravascular volume depletion.
During 2016, the MHRA issued a drug safety update warning about the association between SGLT2 inhibitor use and euglycaemic diabetic ketoacidosis (DKA; MHRA, 2016). It is an uncommon phenomenon (between 1 in 1000 and 1 in 10000 patients) and the MHRA reiterates that the benefits of this class of drug outweigh the risks. However, when commencing an SGLT2 inhibitor, we should warn patients about the symptoms of DKA and, importantly, test for raised ketones in those with symptoms of DKA, even if glucose levels are near normal. The MHRA also reminds us that SGLT2 inhibitors are not approved for use in type 1 diabetes.
Finally, an unexpected finding of the CANVAS trial programme was an increased risk of lower-limb amputations (predominantly toe amputations) with canagliflozin compared to placebo. Absolute risk increase was small, and the highest absolute risk of amputation occurred in individuals with a prior history of amputation or peripheral vascular disease. This triggered a European Medicines Agency (EMA) review of all SGLT2 inhibitors, which concluded that the risk–benefit ratio of SGLT2 inhibitors remains favourable, but did mandate that a warning should be included in the product information of all SGLT2 inhibitors to reflect this finding (EMA, 2017).
The CANVAS trial programme also found an increased risk of fractures with canagliflozin, and the US Food and Drug Administration (2015) recommends a fracture risk assessment when prescribing canagliflozin.
Glucagon-like peptide-1 receptor agonists
SIGN 154 suggests that we consider GLP-1 RAs in those with body mass index (BMI) ≥30 kg/m2 (or ethnically adjusted equivalent) in combination with oral glucose-lowering drugs or basal insulin (or both) as third- or fourth-line treatment, when adequate glycaemic control has not been achieved with these drugs. Furthermore, GLP-1 RAs should be considered as an alternative to insulin in those for whom treatment with combinations of oral glucose-lowering drugs have been inadequate.
GLP-1 RAs are potent glucose-lowering drugs, can lead to significant weight reduction and, overall, have a low risk of hypoglycaemia. The use of GLP-1 RAs is limited by their gastrointestinal side-effects, commonly nausea and anorexia. GLP-1 RAs can be used in severe renal impairment; however, advice for use and dosing varies between the different agents. Further details can be found in the BNF and relevant SPCs.
Of note, liraglutide has proven CV benefit from the LEADER CVOT (Marso et al, 2016). Compared to placebo in a high CV risk population, liraglutide demonstrated significant reductions in CV and all-cause mortality. Furthermore, a small, but statistically significant, reduction in the progression of renal disease was noted. Additionally, LEADER observed a small increase in acute cholecystitis with liraglutide, but, reassuringly, no significant increase in pancreatitis or pancreatic cancer, which had previously been highlighted as a concern for this class of medications. ELIXA (lixisenatide; Pfeffer et al, 2015) and EXSCEL (exenatide once a week; Holman et al, 2017) were two other CVOTs investigating GLP-1 RAs. These trials did not demonstrate any CV benefit, but did establish the CV safety of these drugs.
Once again driven by this landmark CVOT, an additional key new clinical recommendation in SIGN 154 is to preferentially consider a GLP-1 RA with proven CV benefit (currently, only liraglutide) in those with type 2 diabetes and established CV disease.
Insulin
Finally, SIGN 154 offers some pragmatic guidance to healthcare professionals considering insulin when oral agents are no longer effective. Oral metformin therapy should be continued when insulin therapy is initiated to maintain or improve glycaemic control. Once-daily bedtime NPH (isophane) insulin should be used when adding insulin to metformin, and the dose titrated against fasting glucose. If the individualised HbA1c target is not achieved, then addition of a prandial insulin should be considered. Basal insulin analogues should be considered according to risk of hypoglycaemia.
SIGN 154 joins the growing suite of international diabetes guidelines (e.g. Diabetes Canada [2016], American Diabetes Association [2018] and American Association of Clinical Endocrinologists/American College of Endocrinology [2018]) that consider the CV outcomes of antidiabetes drugs, rather than simply their glucose-lowering properties. Cardiovascular disease remains the leading cause of death in those with type 2 diabetes; the key new clinical recommendations in SIGN 154 will help drive improvement in the outcomes of those with type 2 diabetes and established CV disease in Scotland.




Small increases in three lifestyle behaviours is effective in reducing all-cause mortality.
27 May 2025