What is finerenone?
Finerenone (Kerendia®) is a non-steroidal, selective mineralocorticoid receptor antagonist (MRA) used for the treatment of chronic kidney disease (CKD) in adults with type 2 diabetes.
Unlike traditional steroidal MRAs, such as spironolactone and eplerenone, finerenone has a more targeted effect and is thus associated with fewer endocrine-related side effects.1,2
Mechanism of action
Finerenone blocks mineralocorticoid receptors in the kidney, heart and vascular system. This action reduces inflammatory and fibrotic processes, improving outcomes in people with CKD and type 2 diabetes by reducing proteinuria and slowing CKD progression. This effect also provides cardiovascular protection, as shown in clinical studies including FIDELIO-DKD and FIGARO-DKD.1,3
Finerenone selectively blocks mineralocorticoid receptors in the kidney, heart and vascular system. This has the following effects:
- Reduces inflammatory and fibrotic processes.
- Decreases albuminuria.
- Slows CKD progression.
- Provides cardiovascular protection.
Finerenone’s distinctive non-steroidal structure gives it higher selectivity for mineralocorticoid receptors over androgen and progesterone receptors, thus reducing the risk of endocrine-related adverse effects such as gynaecomastia.4
Licensed indication5
In the UK, finerenone is indicated for the treatment of CKD (stage 3 and 4 with albuminuria) associated with type 2 diabetes in adults.
Positioning in guidelines
NICE guidance (TA877)2
NICE recommends finerenone as an option for treating stage 3 and 4 chronic kidney disease associated with type 2 diabetes in adults.
It is recommended only if:
● Albumin:creatinine ratio is persistently ≥3 mg/mmol (30 mg/g), and:
● The person is on optimised standard of care, including (if suitable) the highest tolerated doses of:
- An ACE inhibitor or ARB.
- An SGLT2 inhibitor.
● eGFR is ≥25 mL/min/1.73 m2.
Other guidelines
The KDIGO guideline6 and ADA Standards of Care7 also advocate for the use of finerenone in people with type 2 diabetes, an eGFR ≥25 mL/min/1.73 m2, normal serum potassium concentration and albuminuria despite the maximum tolerated dose of ACEi/ARB.
Principal effects
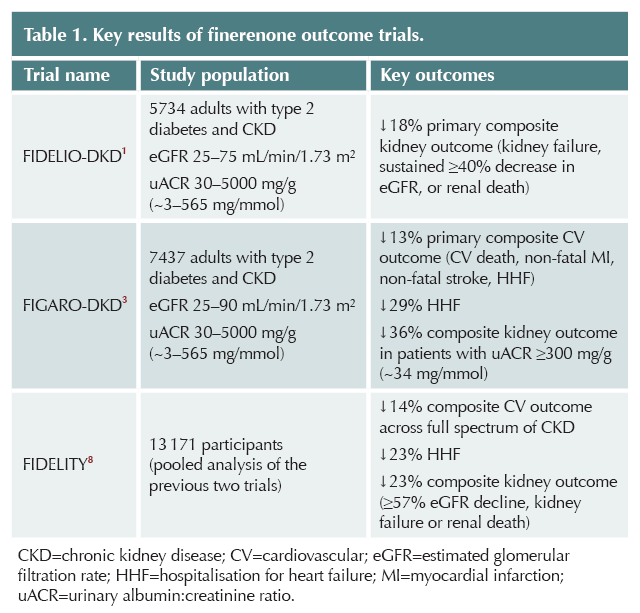
Finerenone has been shown to reduce the risk of renal and cardiovascular outcomes in people with type 2 diabetes and CKD, as shown in Table 1.
Other effects
● Weight: Finerenone has a neutral effect on weight.
● Blood pressure: Modest reduction in systolic blood pressure (2–3 mmHg).
Contraindications
Hypersensitivity to lactose
● Do not prescribe in people with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption.
Interactions with CYP3A4
● Do not prescribe if taking strong inhibitors of CYP3A4 (e.g. itraconazole, ketoconazole, ritonavir, nelfinavir, cobicistat, clarithromycin, telithromycin, nefazodone).
● Do not prescribe if taking strong inducers of CYP3A4 (e.g. carbamazepine, phenytoin, phenobarbital, St John’s Wort.
● Grapefruit or grapefruit juice should not be consumed during finerenone treatment.
Addison’s disease
● Do not prescribe in patients with Addison’s disease (risk of hyperkalaemia).
Severe CKD
● Avoid initiation if eGFR <25 mL/min/1.73 m².
● Discontinue if eGFR is persistently <15 mL/min/1.73 m².
Hyperkalaemia
● Do not start if serum potassium is ≥5.0 mmol/L.
● Finerenone should not be given with:
- Potassium-sparing diuretics (e.g. amiloride, triamterene).
- Other MRAs (e.g. eplerenone, esaxerenone, spironolactone, canrenone).
Hepatic impairment
● Severe hepatic impairment: Do not initiate (lack of data).
Pregnancy and breastfeeding
● Women of childbearing potential should use effective contraception during treatment.
● Avoid in pregnancy unless clinically warranted (no data on use in pregnant women; animal studies show reproductive toxicity).
● Avoid breastfeeding whilst taking finerenone (unknown whether finerenone or its metabolites are excreted in human breast milk; evidence of excretion in animal milk and adverse reactions in exposed offspring).
Cautions
Hyperkalaemia
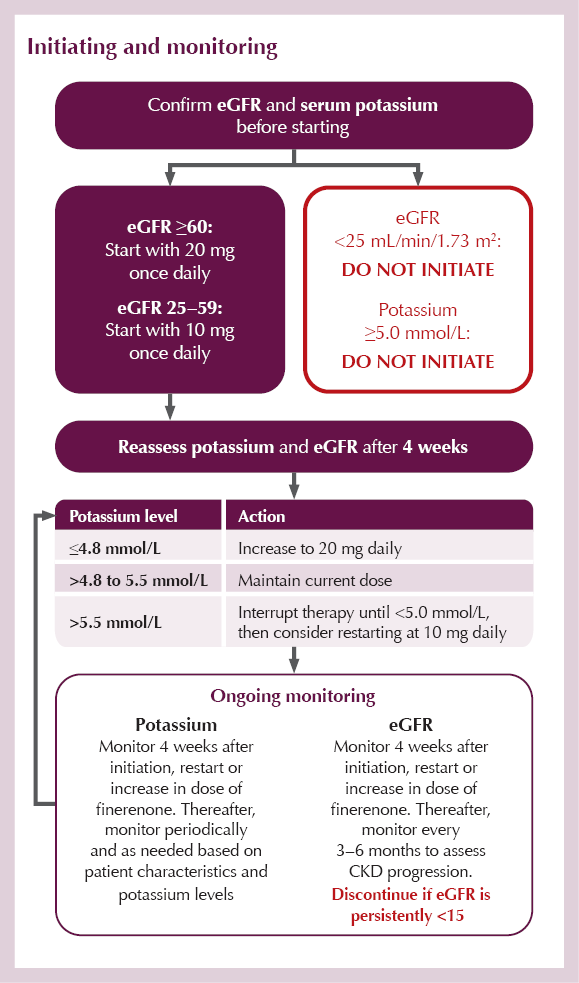
● Patients with a low eGFR (particularly <45 mL/min/1.73 m2)9 are at higher risk of hyperkalaemia and require frequent monitoring (see Initiating and monitoring section).
● Use with caution and monitor serum potassium when taken concomitantly with:
- Potassium supplements.
- Trimethoprim or trimethoprim/sulfamethoxazole (temporary discontinuation of finerenone may be necessary).
● If serum potassium rises above 5.5 mmol/L, finerenone treatment must be withheld (see Initiating and monitoring section).
● Local guidelines for the management of hyperkalaemia have to be followed.
● Once serum potassium falls to ≤5.0 mmol/L, finerenone treatment can be restarted at 10 mg once daily. Thereafter, serum potassium should be remeasured periodically and as needed based on patient characteristics and serum potassium levels.
Hepatic impairment
● Moderate hepatic impairment: No initial dose adjustment required. Additional potassium monitoring should be considered according to patient characteristics, due to an increase in finerenone exposure.
● Mild hepatic impairment: No initial dose adjustment required.
Heart failure
● Patients with NYHA class II–IV heart failure with reduced ejection fraction were excluded from the Phase III clinical studies.
Other antihypertensives
● Hypotension risk increases with concomitant use of multiple other antihypertensives; blood pressure monitoring is recommended.
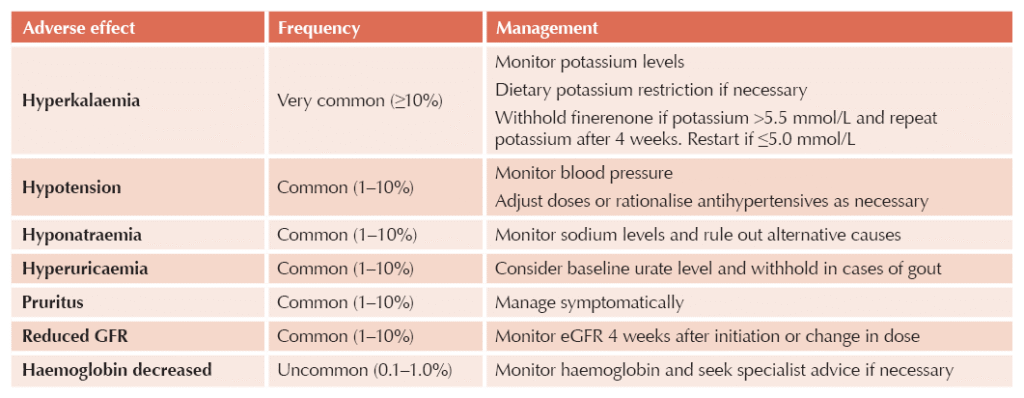
Adverse effects
Initiating and monitoring
Prescribing tips
● The recommended target dose and maximum recommended dose of finerenone is 20 mg daily.
● Tablets may be taken with a glass of water and with or without food.
● Grapefruit or grapefruit juice should be avoided.
● Tablets may be crushed and mixed with water or soft foods, such as apple sauce, directly before oral use.
● Dietary advice: Counsel patients about moderating potassium intake – provide list of high-potassium foods to limit.
● Morning dosing may be preferred, to avoid nocturia.
● NSAID caution: Advise patients to avoid or minimise use of NSAIDs, which can increase potassium levels.
● Medication review: Evaluate all medications for potential potassium-raising effects.
● Sick day rules: Consider temporary withholding during severe illness, dehydration or procedures with contrast media.
● Transition from steroidal MRAs: No washout period is required when switching from spironolactone or eplerenone to finerenone.
● Missed doses:
- A missed dose should be taken as soon as the patient notices, but only on the same day.
- The patient should not take two doses to make up for a missed dose.
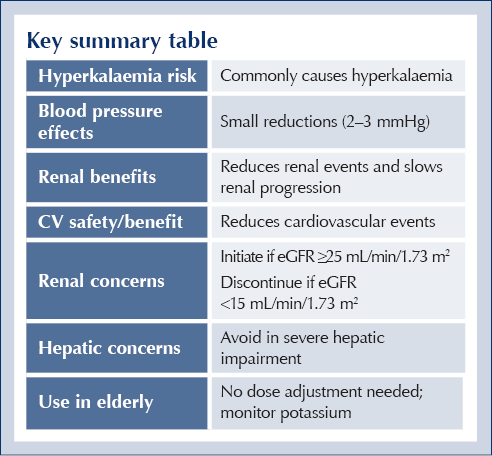
Key summary table




ICBs in England are undergoing a major restructure. Guest editor Hannah Beba asks how this might affect diabetes care.
4 Jul 2025