DAPA-CKD: New data on type 2 diabetes prevention and cardiorenal outcomes
Data from further analysis of the DAPA-CKD study of dapagliflozin for the management of chronic kidney disease (CKD) were presented at the conference.
Type 2 diabetes prevention
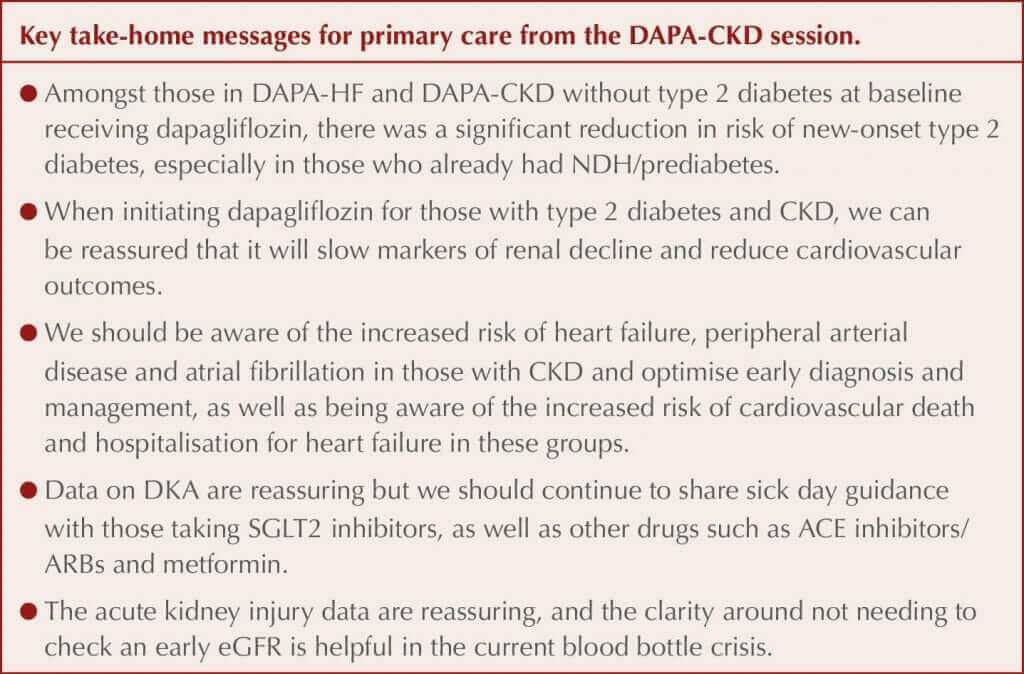
Metabolic data were presented by Professor Silvio Inzucchi (Yale School of Medicine, New Haven, CT, USA). Data previously published from the DAPA-HF study, which included people with heart failure with reduced ejection fraction (HFrEF), with and without type 2 diabetes, demonstrated that dapagliflozin significantly reduced the incidence of new type 2 diabetes by 32% compared to placebo amongst those without diabetes at baseline. Likewise, in the smaller DAPA-CKD study, there was a numerical reduction in the number of people who developed type 2 diabetes during the trial compared to those treated with placebo. A prespecified combined analysis of the two trial populations without diabetes at baseline has therefore been completed.
New-onset type 2 diabetes was defined using the standard definition of an HbA1c ≥6.5% (48 mmol/mol) on two visits. There were 4003 people without diabetes at baseline in the combined study populations; of these, 5.3% developed type 2 diabetes during the trial, and there was a significant 33% relative risk reduction (RRR) with dapagliflozin compared to placebo. When the US definition for prediabetes or non-diabetic hyperglycaemia (NDH) was used (HbA1c 5.7–6.4% [39–47 mmol/mol]), 94% of those who developed diabetes during the trial had NDH at baseline, whereas when using the UK definition of HbA1c 42–47 mmol/mol (6.0–6.4%), 86% had NDH at baseline.
Subgroup exploration identified a larger risk reduction in younger people and women but no difference related to previous cardiovascular disease or medications. For those requiring treatment with dapagliflozin for HFrEF or CKD, decreasing the incidence of type 2 diabetes in those free of the disease can therefore be seen as an additional benefit alongside the significant cardiovascular and renal benefits for which the drug is being used. Dapagliflozin is not currently licensed for diabetes prevention.
This 33% relative risk reduction in new type 2 diabetes in those treated with dapagliflozin compares favourably with reductions achieved in the drug treatment arms in the various diabetes prevention studies; for example, the 31% reductions achieved with metformin in the US Diabetes Prevention Program and Finnish Diabetes Prevention Study. There was no significant difference in HbA1c between those treated with placebo or dapagliflozin, either in those who did or did not develop type 2 diabetes, which is different from what was seen in the drug treatment arms in other diabetes prevention studies. Current theories are that offloading the beta-cells may result in some preservation of beta-cell function, thus reducing risk of type 2 diabetes, but this has not yet been fully assessed.
Renal data
Additional renal data were presented by Professor David Wheeler (University College London). Previous data from DAPA-CKD demonstrated significant reductions in the following:
- Primary composite endpoint: sustained ≥50% reduction in eGFR, end-stage renal disease (ESRD) or death from cardiovascular or kidney disease.
- Key secondary endpoints:
- Kidney-specific endpoint (sustained ≥50% eGFR decline, ESRD or death from kidney disease).
- Cardiovascular death or hospitalisation for heart failure.
- All-cause mortality.
Data from prespecified subgroup analyses of these outcomes were presented. There was a consistent effect on the primary composite renal outcome with dapagliflozin across baseline glycaemic states. Consistent benefits were demonstrated for the primary and all three secondary outcomes across different CKD aetiologies, including amongst people with glomerulonephritides. An important question was whether dapagliflozin would still be effective in those with stage 4 CKD, and a consistent effect with dapagliflozin was demonstrated compared to those with stages 2 and 3 CKD.
All-cause mortality was a prespecified secondary outcome and was significantly lower in those treated with dapagliflozin than placebo in the original trial, with sudden cardiac death and infections as the two main causes of mortality. Further analysis demonstrated that the all-cause mortality benefits appeared to be largely driven by a reduction in non-cardiovascular death (i.e. infections and malignancy), although Professor Wheeler stated this should be seen as hypothesis-generating only as the finding was not expected. The reduction in albuminuria in those treated with dapagliflozin was greater in those with type 2 diabetes than those without.
Acute kidney injury (AKI) was reported as a safety outcome rather than a conventional outcome in the study, and AKI was significantly less in those treated with dapagliflozin compared to controls, despite the initial eGFR reduction that is typically seen when initiating all SGLT2 inhibitors. Professor Wheeler said that this was reassuring and strengthened the case for not checking the eGFR 2 weeks after initiating dapagliflozin, and instead checking this with other blood tests such as HbA1c at 3–4 months. However, if the patient is deemed prone to dehydration then it remains appropriate to check earlier after initiation, and to stop the drug and reconsider if the eGFR has dropped by more than 30%.
Cardiovascular outcomes
Professor John McMurray (University of Glasgow) presented new data exploring the impact of heart failure, peripheral arterial disease and atrial fibrillation in those with CKD, seeking to identify whether these modify the effect of dapagliflozin. All three of these comorbidities occur more commonly in those with CKD and impact morbidity and mortality. The presence of each of the comorbidities was associated with a higher risk of the primary composite outcome, and the secondary composite outcome of cardiovascular death or hospitalisation for heart failure occurred at rates 4–6 times higher in people with CKD who also had one of these conditions. There was no difference in the effects of dapagliflozin on reducing the risk of the primary outcome (overall RRR, 39%) and the secondary cardiovascular outcome (overall RRR, 29%) in those with or without these three cardiovascular conditions at baseline. People with CKD and heart failure, atrial fibrillation or peripheral arterial disease had a much higher baseline risk of cardiovascular death or hospitalisation for heart failure, so the absolute risk reductions with dapagliflozin were large.
Q&As
Professor McMurray suggested that an annual electrocardiogram may be appropriate to diagnose atrial fibrillation in older patients, citing an annual 5–10% incidence in those over 60 years of age. The panel were asked about diabetic ketoacidosis (DKA) and stressed that the incidence rate is very low in those with type 2 diabetes, around 2–3 per 1000 people per year, and more likely in those who are lean or have insulin deficiency, some of whom may have latent autoimmune diabetes in adults (LADA). There were no cases of DKA in the DAPA-HF study even amongst those with type 2 diabetes, and the latest heart failure study only had four cases out of 3000 in the type 2 diabetes group and five cases in the placebo group. The risk of DKA can therefore be put into context as less than the risk of angioedema in those starting an ACE inhibitor or a neprilysin inhibitor.
Asked about when it is appropriate to stop dapagliflozin due to declining renal function, Professor Wheeler reminded the audience that there was no eGFR threshold for stopping the drug in DAPA-CKD and that the results should reassure that this strategy does not seem to cause any harms. Dapagliflozin in the UK is licensed for use down to an eGFR of 15 mL/min/1.73 m2.
TriMaster study: first foray into precision medicine
The TriMaster study tested the feasibility of tailoring second- or third-line glucose-lowering therapy using drugs hypothesised to have different glucose-lowering effects based on BMI or eGFR. 458 people on metformin, with or without a sulfonylurea, with HbA1c 58–110 mmol/mol at baseline, received 12 weeks’ treatment with each of three study drugs (canagliflozin, pioglitazone and sitagliptin). HbA1c and adverse events were evaluated at the end of each treatment, and patient treatment preference was recorded at the end of the study. Advantages and disadvantages of each drug were identified. Patients preferred the drugs that provided their personal best glycaemic control.
Precision medicine can be defined in many ways, but a simple definition is “providing the right therapy for the right patient at the right time”. The American Diabetes Association (ADA) has previously published a consensus on precision medicine in relation to diabetes care, which can be found here. Based on previous studies exploring efficacy of commonly used drugs in people with varying BMIs and eGFR measurements, the investigators began with two hypotheses they wished to test:
- Hypothesis 1. Those with BMI >30 kg/m2 compared to those with BMI ≤30 kg/m2 will achieve a lower HbA1c when treated with pioglitazone than sitagliptin.
- Hypothesis 2. Those with an eGFR 60–90 mL/min/1.73 m2 compared to those with eGFR >90 mL/min/1.73 m2 will achieve a lower HbA1c when treated with sitagliptin than canagliflozin.
Testing the two hypotheses separately would require large numbers of participants, so a three-way blinded, crossover trial design was chosen where each participant was randomised to receive each study drug (canagliflozin, pioglitazone and sitagliptin) for 12 weeks. Data on short-term HbA1c change, adverse events by drug and patient characteristics, and patient drug preferences were collated.
The primary outcome was difference in achieved HbA1c between the therapies in each stratum, while secondary outcomes included weight change, tolerability and patient preference.
Results
Both hypotheses were confirmed during the study, and using the predicted best drug for each person resulted in a mean 3 mmol/mol HbA1c difference between treatment effects in each stratum, although at higher BMIs or different eGFRs individual differences were as large as 10 mmol/mol. Investigators reminded the audience that this was an additional reduction based solely on individualising therapy choice and good prescribing, with no additional drugs or costs. With mean deterioration of HbA1c of 1 mmol/mol per year on stable therapy, this equates to at least 3 years of HbA1c progression.
Although there were no new side effects identified, significant side effects were recorded with each drug, and each drug had advantages and disadvantages.
- Pioglitazone was well tolerated, but resulted in most weight gain.
- Sitagliptin had the fewest side effects, but was poorly tolerated, resulting in more people stopping the drug.
- Canagliflozin was best for weight loss, but had most side effects.
Prior to stating their preferred treatment, participants were informed of their HbA1c reduction with each drug. All preferred the drug that had provided their personalised best glycaemic control, even if this caused significant weight gain (pioglitazone) or side effects.
The investigators highlighted that this is the first trial to test and prove a stratified approach as a primary outcome, rather than as a secondary outcome or in subgroup analyses. This study used routine clinical data (BMI and eGFR) to accurately predict differential drug response (i.e. that despite drugs having similar glucose-lowering efficacy, specific drugs work better in specific patients). They concluded that when glycaemia is the priority, in patients with BMI >30 kg/m2 glitazones effectively lower glucose in the short term, but at the cost of increased weight. DPP-4 inhibitors work well when BMI is <30 kg/m2. Despite the study’s findings, the researchers stressed that this does not mean that all patients with a BMI >30 kg/m2 should have pioglitazone.
The protocol for this study was designed prior to the publication of the first cardiovascular outcome trial of an SGLT2 inhibitor and, therefore, glycaemia and adverse events remained the most important parameters for choosing and improving type 2 diabetes care. Therefore, cardiovascular profiles were not included in the protocol and pre-existing cardiovascular disease, heart failure and chronic kidney disease (CKD) would now inform drug choice in preference to glycaemia for many with type 2 diabetes, as per the ADA/EASD glycaemic consensus.
5–9% of participants stopped taking medication in the first 12 weeks due to side effects, and DPP-4 inhibitors were discontinued most commonly, despite fewer apparent adverse effects being recorded. After trying all three drugs, 25% preferred pioglitazone, 35% sitagliptin and 38% canagliflozin. Patients preferred the drug that provided their individualised best glycaemic control, with no clear influence of increased weight.
This study raises the question of whether patient-centred diabetes care should allow patients not just to participate in drug choice, but to have the option to trial two or three drugs that fit their clinical characteristics, to identify their personal preference.
Professor Caroline Kistorp (University Hospital Copenhagen), providing the commentary, congratulated the investigators for moving precision medicine forward into evidence-based diabetes therapy. She commented that it would have been clearer if all participants had only been on metformin at baseline, rather than some receiving a sulfonylurea, and felt it would have been useful to study a GLP-1 receptor agonist, rather than pioglitazone. The investigators reminded her that pioglitazone remains an important drug for treating some groups of patients in the UK and is widely used in other parts of the world, and therefore remains important to study.
For those with cardiovascular disease, CKD or heart failure, personalisation of medication choice is undertaken based on the 2019 update to the ADA/EASD consensus report, but individualisation based on glycaemia is still important for other groups. The TriMaster results were presented at the 57th EASD Annual Meeting by Andrew Hattersley, Ewan Pearson, Catherine Angwin and Beverley Shields.
GRADE study: a head-to-head drug comparison
GRADE (Glycaemia Reduction Approaches in Diabetes: a Comparative Effectiveness Study) was a head-to-head comparison of second-line (after metformin) use of the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin, the sulfonylurea glimepiride, the glucagon-like peptide-1 receptor agonist (GLP-1 RA) liraglutide and insulin glargine in people with <10 years’ type 2 diabetes duration (mean 4.2 years in those recruited), who were followed for an average of 5 years. The study was also designed to inform whether different drugs were more or less effective in different sub-groups and hence how personalised or individualised prescribing might be achieved. Choosing to include drugs that were already licensed in the US and for which there was significant experience of use meant the study results were designed to allow immediate implementation after completion.
Preliminary data were shared at the American Diabetes Association 81st Scientific Sessions in June and included in this journal’s report of the conference. Here, we provide an overview of the methodology and of the final microvascular and macrovascular results, which remain unpublished, so no graphical illustrations can be included here.
The primary outcome was the time to an HbA1c ≥7% (53 mmol/mol), confirmed with a second HbA1c at the next clinic visit. The secondary outcome was time to confirmed HbA1c ≥7.5% (59 mmol/mol), after which insulin glargine was to be added to the 3 groups who had not received it previously. The tertiary outcome was time to another HbA1c of ≥7.5% (59 mmol/mol) after the secondary outcome, at which point the randomised drugs were stopped and insulin therapy was intensified to control glycaemia. Each outcome HbA1c had to be confirmed at the next clinic visit 3 months later. Drugs were titrated according to their labelling, and sitagliptin dosed as per eGFR recommendations.
Results presented previously demonstrated that the two injectable therapies provided improved ability to keep HbA1c <7.0% (53 mmol/mol) for longer, while liraglutide and sitagliptin were associated with more weight loss than the other two drugs. As anticipated, liraglutide had the most gastrointestinal side effects and glimepiride was associated with the most hypoglycaemia.
Sodium–glucose cotransporter 2 (SGLT2) inhibitors and thiazolidinediones (TZDs) were not included in the study since, at its inception, the first SGLT2 inhibitor had not yet been licensed by the FDA, and the TZD class was suspected of causing significant side effects and was not appropriate to consider for a long-term study.
Microvascular results
As the participants had relatively short duration of type 2 diabetes, microvascular complication rates were low:
- Moderately increased microalbuminuria (>3.39 mg/mmol): 12%
- Severely increased microalbuminuria (>33.90 mg/mmol): 5%
- eGFR <60 mL/min/1.73 m2: 12%
There were no significant differences between the treatment groups in any of the microvascular complication rates.
Macrovascular results
Only 6% had established cardiovascular (CV) disease at baseline, and people with NYHA class 3 or 4 heart failure or with a major adverse CV event (MACE) in the preceding 12 months were excluded from the study.
There were no significant differences in development of hypertension or hyperlipidaemia between treatment groups in GRADE. All CV events were adjudicated as per recommendations.
Cardiovascular endpoints included:
- 3-point MACE (first occurrence).
- Any CV outcome “6-point MACE” (MACE event, hospitalisation for heart failure, unstable angina requiring hospitalisation or revascularisation, or revascularisation or repair in any vascular bed).
- Hospitalisation for heart failure.
- CV death.
- Total mortality.
The so-called “6-point MACE” broad CV outcome demonstrated a statistically significant lower risk with liraglutide (6.6% of events), with no significant differences between the other three drug groups (8.9%–9.5%). For paired comparisons, liraglutide-treated groups had a significant 32% fewer any CV outcomes than those treated with sitagliptin, and 29% fewer than the glimepiride-treated group. There was no significant difference in relation to the glargine group, although it was trending in the same direction. None of the other pairings were significant, so were not presented. There was no heterogeneity in the relative risk of the broad composite “any CV outcome” depending on whether baseline CV disease was present or absent, although absolute event rates were higher in those with pre-existing CV disease.
Although there were numerically fewer 3-point MACE events, hospitalisations for heart failure, CV deaths and total deaths in those treated with liraglutide, these did not reach statistical significance for any of the outcomes, and there was no difference between the other three groups.
Serious adverse events were generally low, with no differences between groups. Severe hypoglycaemia requiring assistance was infrequent, but was higher in the glimepiride group. Pancreatitis and pancreatic cancer were very low, and no differences were reported between groups.
Mean weight at 1 year was significantly lower with liraglutide and sitagliptin, and weight gain of >10% across the whole study period occurred less with liraglutide compared to the other treatment groups, with no significant difference between them.
Providing the independent commentary, Professor David Matthews (University of Oxford) congratulated the study organisers for a well-conducted trial. His further comments were similar to those he made during his presentation at the ADA conference in June, as reported in this journal. He challenged the fact that participants had had type 2 diabetes for differing durations, up to 10 years at baseline, as well as the failure to include TZDs and SGLT2 inhibitors, the use of glimepiride rather than gliclazide, and the fact that the study was undertaken in a US population only. He highlighted that although hypoglycaemia numbers were small, they were 2–3 times more common in those treated with glimepiride than with liraglutide or sitagliptin. He concluded that GRADE was a good study, but that there were very few findings to guide individualisation of therapy.





Risk ratios of 1.25 for autism spectrum disorder and 1.30 for ADHD observed in offspring of mothers with diabetes in pregnancy.
18 Jun 2025