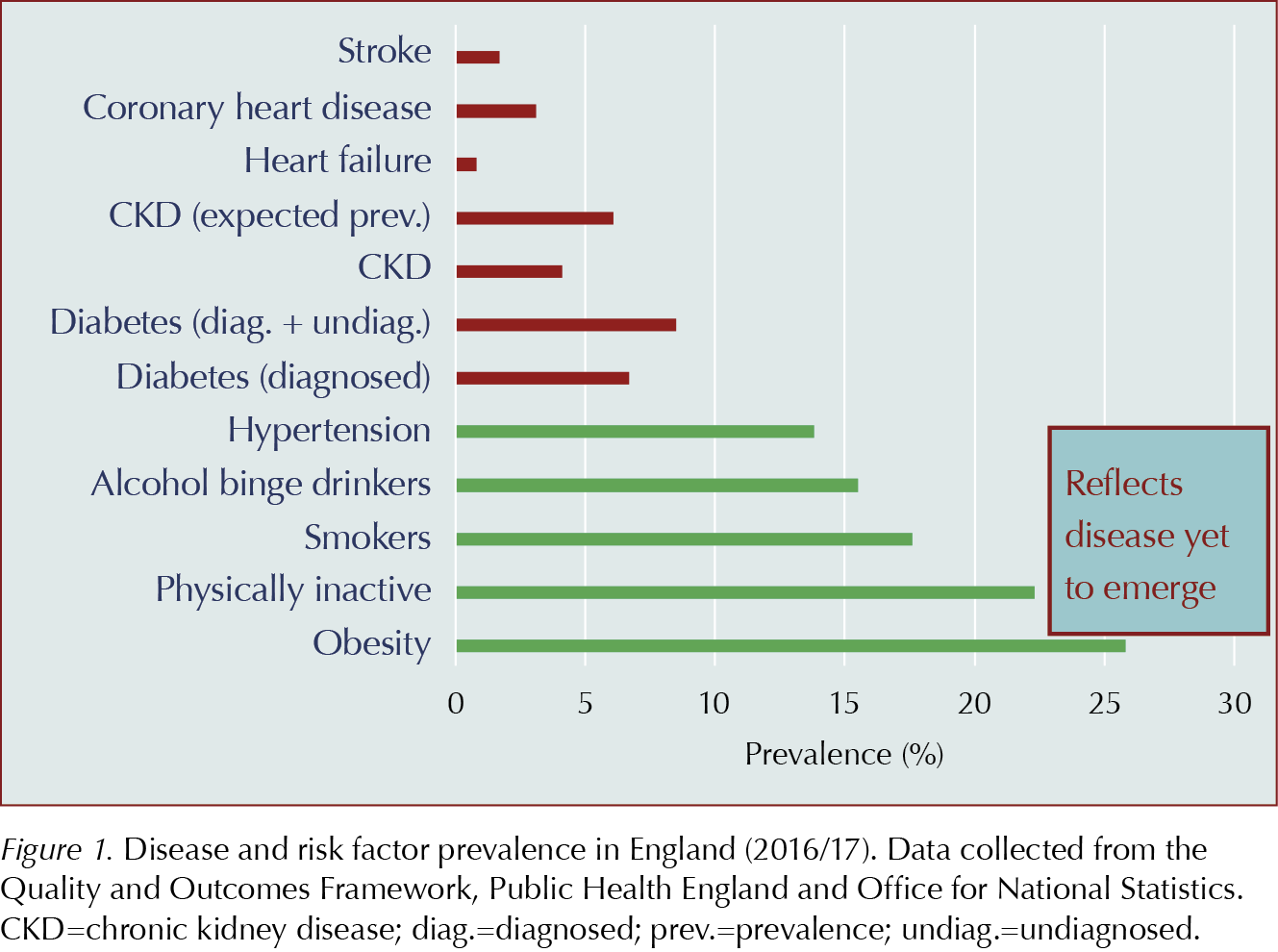
In 2018, around 3.8 million people were living with diabetes in the UK, with estimates suggesting a further one million people may have undiagnosed diabetes (Diabetes UK, 2018). But type 2 diabetes rarely presents alone: a retrospective study of comorbidities linked with type 2 diabetes reported that 97.5% of individuals with the condition had at least one comorbid condition and 88.5% had at least two, with the risk increasing in older age groups (Iglay et al, 2016). Hypertension was the most common comorbidity, found in 82.1%, followed by overweight/obesity in 78.2% and hyperlipidaemia in 77.2%. Chronic kidney disease was found in 24.1% and cardiovascular disease in 21.6%. Hypertension and hyperlipidaemia coexisted in 67.5% of patients with type 2 diabetes.
These statistics reflect that clusters of lifestyle risk factors underpin many long-term conditions (LTCs), and social deprivation as well as genetic factors can compound these clusters. Deprivation is strongly linked with obesity rates (Public Health England, 2017), physical activity levels (Farrell et al, 2013), smoking (Wise, 2014) and dietary quality (Whybrow et al, 2018).
Figure 1 compares the prevalence of common risk factors and diseases. However, health service targets (e.g. Quality and Outcome Framework [QOF] workload) and treatment costs show far greater NHS focus on treating established disease than in tackling risk factors, despite compelling economic arguments (Ferguson, 2016).
Times are gradually changing, with more investment in some community prevention services alongside increasing public acceptance of the need to tackle lifestyle factors. But health behaviours reflect a complex set of choices, not just availability of services or financial security; more comorbidities generate more barriers to adopting lifestyle change.
Importantly, the accessibility of local services may impact on the deprivation gap due to the greater agency of those of higher socioeconomic status promoting greater engagement, whilst the hard to reach remain unreached (Pampel et al, 2010).
Care burden is additional to illness burden
Studies have assessed how much the effort of looking after health and the care processes themselves add to the burden of the illnesses. With accumulating comorbidities, the care and treatment burden (such as ordering, collecting and taking complex medications, undertaking self-monitoring of conditions and attending multiple appointments) also accumulates (Eton et al, 2012).
This burden also affects care-givers, with pressure to focus on multiple agendas during short consultations and increasing complexity of management decisions (e.g. from polypharmacy creating drug interactions or from comorbidities generating contra-indications to standard management options). Guidelines that focus on a single condition may feel inappropriate for someone with several long-term conditions that create more complex health priorities (NICE, 2016). The evidence for recommendations in NICE guidance on single health conditions is regularly drawn from people without multimorbidity who are taking fewer prescribed regular medications. The NICE multimorbidity guideline gives permission to take a holistic perspective and to bear in mind feasibility aspects of care as well as personal choice after informed decision making (NICE, 2016).
Emerging evidence
Weight control is well recognised as a fundamental element of reducing type 2 diabetes risk and improving established diabetes control. Approximately 90% of people with type 2 diabetes are overweight or obese (World Health Organization, 2019). Evidence for weight reduction and comorbidity improvement is now strong enough for bariatric surgery to be considered a first-line option where BMI is >35 with comorbidities. It can be considered at lower BMI levels if type 2 diabetes has been recently diagnosed, or for some ethnic groups. Diabetes can rapidly go into remission after bariatric surgery, even before significant weight reduction occurs (Ionut and Bergman, 2011). Mechanisms go beyond calorie restriction, and may involve alterations to the gastrointestinal hormones that play a role in energy homeostasis and to vagal nerve innervation. Whilst results can be impressive, relapse may occur and careful multidisciplinary assessment, and pre-operative counselling about the realities of bariatric surgery and the need for long-term follow-up, is essential (Maciejewsko et al, 2016).
However, surgery may not be appropriate or wanted by the majority of people with obesity, even if surgical capacity was available. Alternative approaches are being evaluated and promising results have been shown from structured weight management support using meal-replacement drinks in primary care settings. The DROPLET study confirmed that total diet replacement programmes are acceptable and feasible in primary care settings, with results showing a 10.7-kg mean weight loss from a 12-week programme (Astbury et al, 2018). The DiRECT trial recruited participants with onset of type 2 diabetes within the previous 6 years and BMI between 27 and 45. Randomisation was to either best-practice care or very-low-calorie meal-replacement drinks for 12 weeks, with on-going support for 12 months. Remission of diabetes was achieved by 46% of the treatment group who achieved a mean weight loss of 10 kg, whereas the control group achieved a mean weight loss of 1 kg, with 4% remission of diabetes (Lean et al, 2018). Remission was sustained 2 years later in over a third of participants (Lean et al, 2019).
Physical activity (in conjunction with, rather than instead of, dietary and medication approaches) has been shown to benefit a variety of indicators for adults with type 2 diabetes, such as glycaemic control, lipid profile, body composition, quality of life and vascular health (Madden, 2013). Whilst the ideal “exercise prescription” remains unclear, safe recommendations for older adults include being active for around 150 minutes per week and to include some muscle-strengthening activity (Chief Medical Officers, 2011).
Building engagement in behaviour change
LTCs may add a significant burden to a patient’s motivational drive. In spite of escalating benefits of behaviour change when several comorbidities are present, barriers also mount. Barriers may arise from pain, fatigue, immobility, isolation or financial constraints, but may also stem from fading confidence that any effort put in will generate meaningful benefit.
Motivational interviewing (MI) techniques aim to unlock solutions from within the patient, whereas in traditional approaches the health professional tends to recommend solutions. MI is particularly useful when the barriers the patient perceives may be unclear or complex, such as when physical problems are accompanied by psychological pressures or social and domestic difficulties.
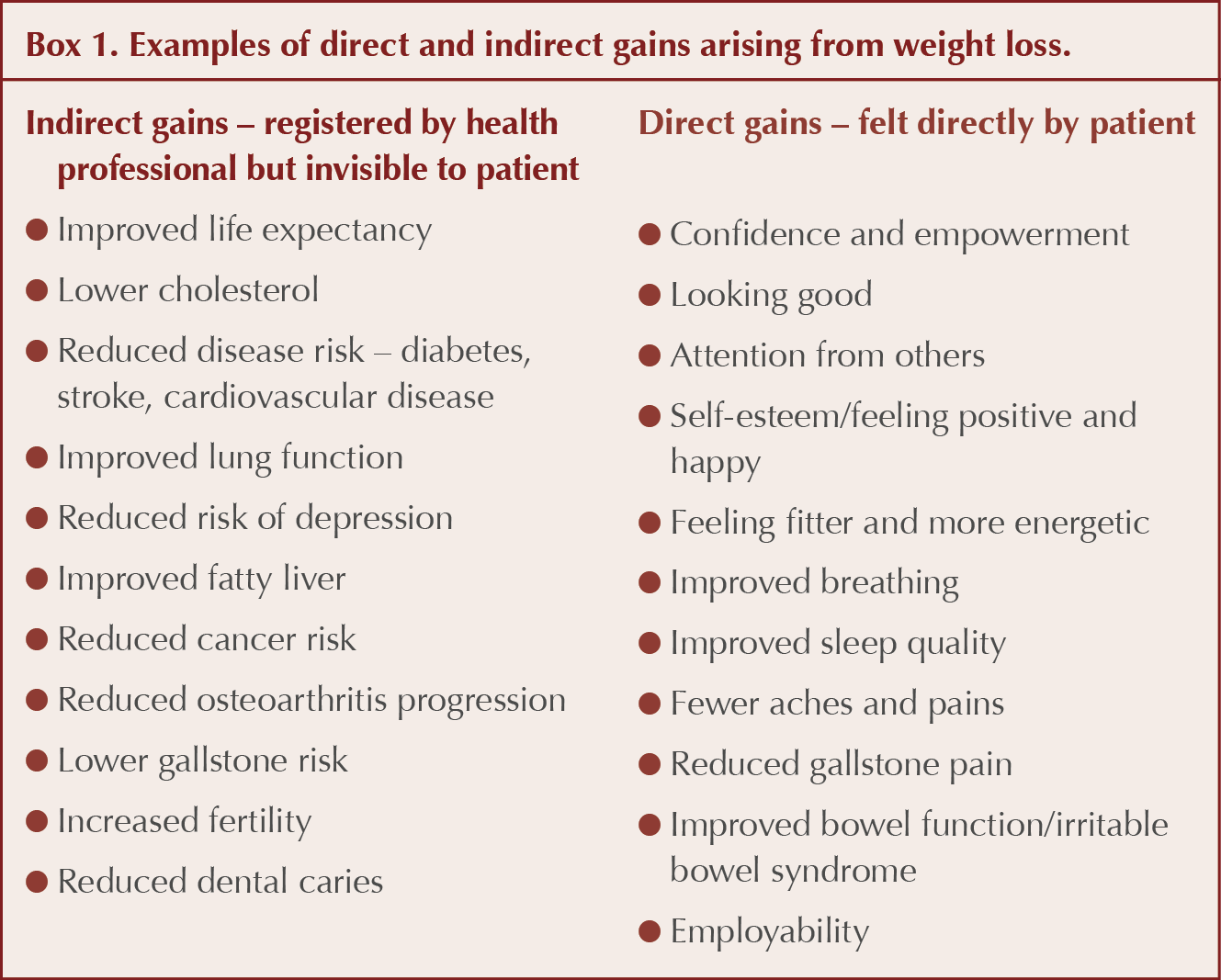
When helping a patient with several conditions to explore the motivation required for behaviour change, it is important to recognise that the patient’s goals and priorities may be markedly different from those of the health professional. Patients are more likely to perceive and gain motivational support from “direct” tangible gains, whereas health professionals may have incentives to focus on “indirect” gains (such as long-term future risk of a condition), which, whilst beneficial across a population, may be imperceptible to the individual and hence have limited motivational impact. Box 1 gives examples of direct and indirect gains arising from weight loss.
Warning patients of dire consequences of inaction, such as explaining risks of disease progression, loss of limbs or vision, or even risk of death, may paradoxically trigger denial rather than engagement, particularly if the barriers to solving the problem make it feel insurmountable. Whilst ensuring patients are appropriately informed of relevant risks, it is important to balance this with a sense of hope and realism about what the patient feels they can achieve, rather than advocate a counsel of perfection.
By asking which issues matter most to the patient, and then building the conversation around potential solutions that the patient may already have in mind, the patient’s own plans for behaviour change can be positively linked to a variety of direct gains. These can help to drive a virtuous motivational circle – a recurring pattern where the result of each determined step increases the beneficial effect of the next. For example, feeling more confident from initial weight loss may boost motivation to increase physical activity. In comparison, any impact of altering diet to lower cholesterol will remain unseen unless blood testing is undertaken.
When introducing important concepts that can improve diabetes control but which may feel challenging, such as increasing physical activity for someone with limited mobility, it is important to present advice carefully. Box 2 outlines two alternative approaches to discussing physical activity. The first example gives a traditional “solutions presented by the health professional” approach, which triggers an ambivalence response from the patient. Despite knowing it would be helpful, the patient responds to each suggestion with escalating reasons why they will not work, culminating in frank refusal.
The second example begins by presenting evidence in the third person – a useful way to provide information without implying “You should”. By asking the patient’s attitude to physical activity, the conversation quickly establishes that the patient would take a positive attitude if the barrier she is concerned about – deterioration in her knee arthritis – could be addressed. The result is that the patient requests help rather than turns help down.
Coordinating care for people with several long-term conditions including diabetes
A major step forward to streamline care and reduce care-burden would be to create combined chronic disease clinics, to enable the patient’s comorbidities to all be reviewed at the same time. Whilst organisational factors may make this challenging, effective and feasible case examples are emerging.
The House of Care Framework is an example of coordinated and integrated care, focusing on delivering person-centred care that addresses how to help patients live well with their condition (NHS England, 2019). Gateshead adopted a successful framework across their CCG with funding to support staff training. But individual practices can also make positive changes, such as by developing merged templates (e.g. using Ardens templates; https://www.ardens.org.uk) or by coordinating patient blood tests before review appointments using recall systems such as PatientChase (https://www.emishealth.com).
Not only should the mechanics of care be considered, but also treatment aims from a holistic perspective. The International Diabetes Federation (2013) published a global guideline outlining more relaxed targets for older people with type 2 diabetes and functional limitations, to create a more pragmatic set of guidelines where strict targets (e.g. for HbA1c, blood pressure and lipid levels) are not in the best interest of the patient. Using the Frailty Index (newly incorporated into the new QOF diabetes targets for 2019/20) can help with developing a holistic understanding of the patient’s care needs and health priorities. Involving patients in making decisions about their own care, as well as in the design of care pathways that bridge across different combinations of comorbidities, will ultimately benefit the whole system of care.





Jane Diggle discusses emotional health and diabetes distress, and offers some tips for discussing this in our consultations.
11 Nov 2025