Twenty years ago, insulin pump therapy was starting its rehabilitation in UK clinical practice and the first continuous glucose monitor, from Medtronic, became available. The insulin pumps were reliable, with sophisticated technology integrated to ensure that pump malfunction was identified at an early stage, and this provided reassurance given the experience in the UK in the early 1980s, when pump failure had resulted in significant morbidity and even mortality. However, there was little flexibility in pump insulin delivery – only one basal rate profile could be programmed, although a different rate could be programmed each hour, and only a standard bolus dose could be delivered. Continuous glucose monitoring (CGM) was even more rudimentary – accuracy was limited, the information could only be reviewed retrospectively, so there were no real-time alarm functions, and the downloaded data display was in black and white!
Now insulin pump therapy has advanced substantially, with different types of pumps and more advanced insulin delivery systems, integrating bolus calculator algorithms. Indeed, the potential for further innovation in pump technology appears limited, so that the selling point for new pumps may come down to user acceptability, with ergonomic considerations, such as size, weight and even colour, increasingly important. Technological advances related to pump therapy focus on communication with the Cloud to allow easy data upload and information-sharing with family, friends or healthcare professionals, as well as integration with CGM to allow automation of insulin delivery, resulting in hybrid closed-loop systems such as the Medtronic 640G and 670G, and the still-evolving fully closed-loop systems.
In contrast, CGM is only now coming of age as a valued technology for people with type 1 diabetes. The accuracy of currently available systems allows confidence in decision-making based on real-time blood glucose data. Alarmed real-time systems provide reassurance for those with problematic hypoglycaemia; however, the FreeStyle Libre, using the same glucose-sensing technology without any alarm function and relying on intermittent scanning to collect the continuous glucose data, has provided a significantly cheaper alternative, making glucose-sensing technology tantalisingly close to a replacement for capillary blood glucose monitoring in a large proportion of people with type 1 diabetes.
This article reviews the currently available technologies and their limitations, and discusses how healthcare professionals should go about comparing them.
Insulin pump therapy
Table 1 shows the latest versions of the main insulin pumps currently available in the UK. Other players (e.g. Medtrum, Tandem) are starting to gain a presence in the UK market, and others (e.g. Kaleido) are likely to do so in the near future. While there is still a small number of Animas users in the UK, the parent company, Johnson & Johnson, plans to completely withdraw Animas from the market by September 2019.
An estimated 15–20% of people with type 1 diabetes in the UK are using insulin pump therapy, and the majority of these pump users are on Medtronic or Omnipod systems (NHS Digital, 2018). The Dana pump has had an increase in popularity as it can be used in OpenAPS systems, which use open-source software to link CGM data with the pump to create a user-built, fully closed-loop insulin delivery system.
Table 1 was created to show the characteristics of an insulin pump that might be thought important by users or their healthcare professionals when choosing the most suitable pump for them. The initial choice lies between a tethered pump or a patch pump. It is notable that new pumps coming to the market are invariably patch pumps, and this probably reflects a user preference for the convenience of this type of device, provided it delivers the same capabilities and reliability as a tethered pump. The Medtronic system will still be preferred by pump users who want to retain the ability to use sensor-augmented pump therapy. Automation of insulin delivery is a particularly attractive feature of the Medtronic pump and CGM systems, and their latest system, the 670G, will not only shut off insulin delivery when hypoglycaemia is predicted to occur within a short time frame (as the 640G does), but will also adjust basal infusion rates against sensor glucose levels, aiming to keep glucose levels to a pre-set target. There is no system currently available that integrates a patch pump with CGM to allow automation of insulin delivery, but such systems are in development.
Patch pumps have the advantage of being smaller and weighing substantially less than a tethered pump, as the technology that controls insulin delivery is integrated into the separate handset, and by having the infusion set integrated into the pump (as in the Omnipod) or as small piece of adjacent tubing (Cellnovo), users do not have the inconvenience of a length of tubing to conceal. However, there is a risk that the separate handset can be lost or left behind when going elsewhere. Under such circumstances, with no means of controlling it, the pump can only dispense pre-programmed basal insulin. Handsets have not always been as ergonomic as might be expected, but the latest Omnipod and Cellnovo controllers are based on an Android phone platform and so look like a contemporary mobile phone device, albeit without any other function beyond controlling the pump and storing data related to pump therapy.
In terms of pump features related to insulin delivery, either basal infusion rates or bolus insulin dosing, there is little difference between any of the available pumps. For example, it is unlikely that a pump user would need to store more than four or five basal rate profiles or use a bolus dose extension lasting beyond 8 hours. All pumps have bolus calculator software and, whilst the algorithms used may vary, there is no evidence they perform substantially differently.
The frequency of the basal insulin pulses that create the “continuous” infusion does vary, and this can be important when insulin infusion has been suspended, as a lower frequency of pulses will result in a longer delay to restoration of insulin delivery, but this will only be clinically significant when very low infusion rates are used, such as in very young children. Similarly, the ability to deliver very low basal rates and very small incremental changes in basal rates will be most important in this population. In this respect, the tethered pumps are superior to the patch pumps. The time for an occlusion alarm to be triggered is again volume-dependent, and so will take longer at low infusion rates. It would be helpful if there were a standardised method for describing this feature, as some pump users may want reassurance as to how pumps compare in this regard.
One area where pump performance might be expected to improve is their longevity and reliability. Recent studies from New Zealand and Italy separately concluded that the mean life expectancy of a pump was just over 2.9 years (Ross et al, 2016; Rabbone et al, 2017). However, in almost one third of cases, pump failure resulted from accidental damage, either cracking or water entry. There is definitely room for improvement in terms of the infusion sets for tethered pumps, which show evidence of occlusion after 3 days’ usage for most pump users (Patel et al, 2014).
Continuous glucose monitoring
Table 2 shows the latest versions of the main continuous glucose sensors available in the UK. As with pumps, other players are entering the market, and again one of these is Medtrum, which has an as yet unproven hybrid closed-loop system similar to Medtronic’s 640G.
There is considerably greater variation between CGM systems than between pumps, not least in terms of cost. The FreeStyle Libre sensors cost £910 per year (to the NHS), whereas the other frequent-wear sensors cost around £2500 per year, and the Eversense implantable sensor costs around £3000 per year.
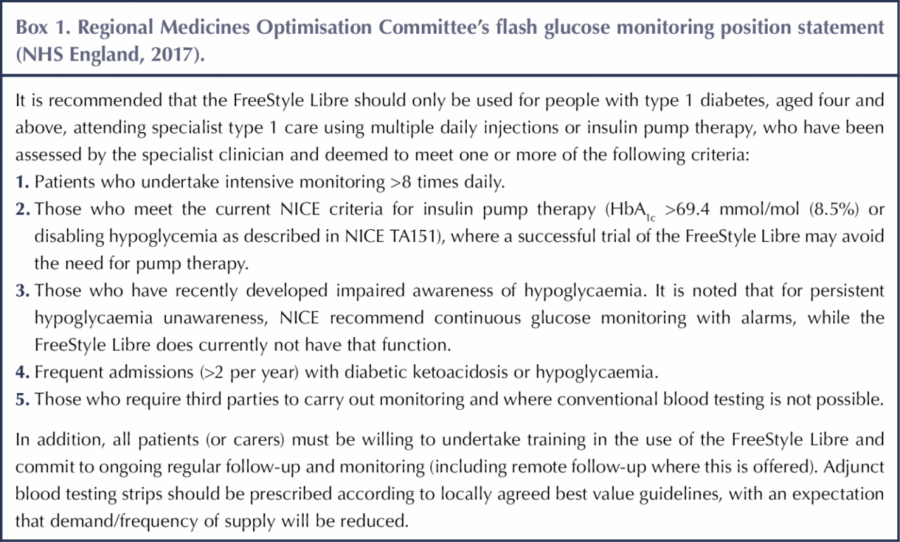
For self-funding CGM users, the FreeStyle Libre is an attractive option on cost grounds but does not provide the security of alarms, which are particularly important for users with impaired awareness of hypoglycaemia. Fortunately, most areas now have a policy for providing NHS funding for CGM based on the hypoglycaemia criteria outlined in NICE Guideline NG17 (NICE, 2015), so those with impaired hypoglycaemia awareness or frequent severe hypoglycaemia should be able to access this technology, which can be life-changing as a result of the protection it provides. In contrast, it has been more challenging to secure NHS funding for the FreeStyle Libre, with those areas where funding is available mostly using the Regional Medicines Optimisation Committee funding criteria (Box 1; NHS England, 2017).
CGM technology is relatively fast-moving, and all sensors can communicate wirelessly with a smartphone to allow display of glucose data. One particular feature that made the FreeStyle Libre stand out was the fact that it does not need calibration from capillary blood glucose tests. This has meant the cost of the sensor for the NHS can be offset by the savings on glucose meter test strips. In addition, the recently released Dexcom G6 sensor is the first real-time CGM that does not require calibration, setting it apart from the Medtronic Enlite 3 sensor. Furthermore, the G6 is marketed for 10-day use, up from 7 days with previous sensors, which reduces annual sensor cost.
The alarm and alert features associated with all the real-time CGM devices (i.e. not the FreeStyle Libre, which depends on intermittent scanning unless used with add-on technology such as the MiaoMiao) do not differ significantly; all have threshold, trend and predictive functions, so it is improvements in sensor life and accuracy that will be particularly important in determining which systems are favoured.
Sensor accuracy is usually defined by using the mean absolute relative difference (MARD) from reference blood glucose levels, although there are flaws with this particular method of assessing accuracy (Ajjan et al, 2018). The figures quoted in Table 2 are those provided by the manufacturers, but they can be based on the difference between the sensor glucose measurement and either a capillary blood glucose reading or a venous blood glucose laboratory assay result, so the MARD values are not necessarily comparable. There is also considerably more error when blood glucose levels are in the hypoglycaemic range; however, this variation in error is masked by the use of an average across all blood glucose levels. And finally, a MARD of 10% might mean that one sensor could show no difference from the reference blood glucose readings whilst another showed a 20% difference. Therefore, when considering accuracy, it is important to stress to users that the most useful information they get from continuous glucose sensing, and indeed from the FreeStyle Libre, may well be the trends in glucose levels rather than the absolute values. This explains why, despite potential inaccuracies in the sensor, the Medtronic SmartGuard system, where a predictive low-glucose insulin-suspend function is activated, prevents 85% of hypoglycaemic episodes (Choudhary et al, 2016), because it depends as much on the trend as the absolute glucose value to activate suspension of insulin delivery.
Future developments
Insulin pumps and CGM are now well-established technologies for the management of type 1 diabetes in the UK. The future direction of innovation for these technologies appears predictable – for pumps, improvements in the user interface with more ergonomic design and wireless transmission of pump data to the Cloud will be targeted; for CGM, improvements in sensor life and accuracy will be the aim. The additional goal will be to bring the technologies closer together, with systems evolving from the currently available hybrid closed-loop to fully closed-loop insulin delivery. As these technologies evolve, it will be crucial for healthcare professionals to remain alert to the features that are most important to the user, and to be able to select the right technology solution for each individual based on these features.






Study provides new clues to why this condition is more aggressive in young children.
14 Nov 2025