Prediabetes is a term that is being increasingly used in replacement of borderline diabetes, both seemingly being used to create a label when describing specific population groups (Harding, 2014). The term refers to abnormally high levels of blood glucose that are not yet at the diagnostic threshold for diabetes. The World Health Organization (WHO, 2006) discourages the terms prediabetes and borderline diabetes because high risk does not mean that a diabetes diagnosis is inevitable, and the terms can potentially increase the perceived stigma associated with a diagnosis. Diabetes UK (2015) also advocates against the terms owing to lack of clarity over their meaning, instead agreeing with the WHO and the phrase “at high risk of type 2 diabetes”. Despite this, in recognition of its widespread usage, the term prediabetes will be used in this article; however, these concerns should be borne in mind when reading.
Evans et al (2007) highlighted that a third of all people diagnosed with prediabetes will develop type 2 diabetes within 6 years. Diabetes UK (2014a) states that between 5% and 10% of all people diagnosed with prediabetes will develop type 2 diabetes each year. This figure is alarming as approximately 18 million people in the UK potentially have prediabetes. The International Diabetes Federation (2017) has predicted that the prevalence of prediabetes globally could be as high as 531.6 million people by 2045.
This article aims to provide an overview of prediabetes and its diagnostic criteria, and to explain why targeted interventions and support are important in this high-risk group.
Diagnostic criteria
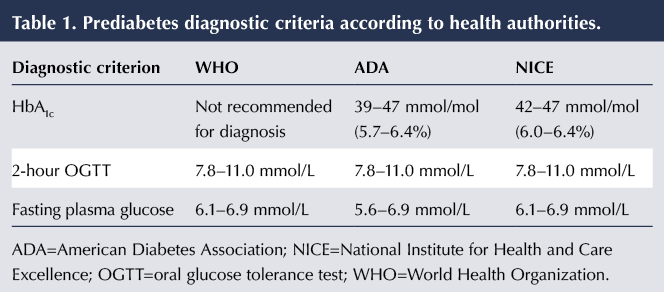
Prediabetes can be defined in terms of either impaired glucose tolerance (IGT), impaired fasting glucose (IFG) or HbA1c. The diagnostic criteria differ between WHO, NICE and the American Diabetes Association (ADA). These differences are summarised in Table 1. WHO (2006) does not advocate the use of HbA1c to diagnose prediabetes, while ADA (2014) and NICE (2012) have set differing levels to define the condition. All three organisations do, however, agree on the definition of IGT after a 2-hour oral glucose tolerance test.
Further variation occurs with regard to IFG, with both WHO (2006) and NICE (2012) defining a fasting plasma glucose level of 6.1–6.9 mmol/L as prediabetes, whereas ADA (2014) specifies the range as 5.6–6.9 mmol/L. WHO (2006) investigated the relevance of this discrepancy and concluded that there was a lack of evidence regarding the benefits of using the lower end of the range, as the population group in the 5.6–6.0 mmol/L range has half the risk of developing diabetes as those in the higher range. The ADA chose to use the lower cut-off point to ensure that the prevalence of IFG was similar to that of IGT (ADA, 2010). The ADA expert committee believed that, by doing so, there was a potential to reduce mortality and the incidence of cardiovascular disease by targeting lifestyle and dietary interventions in higher-risk groups earlier.
Risk factors for prediabetes
The likelihood of developing prediabetes and diabetes may depend on a combination of genetic, lifestyle and environmental factors. As is widely reported in the media, obesity is the major contributor to the likelihood of developing these conditions, with 80–85% of cases linked to obesity (Public Health England, 2014). As obesity is often preventable, it has become a world public health priority.
Other common influencing factors include an individual’s family history (people with a first-degree relative with type 2 diabetes are two to six times more likely to develop the condition than those without) and ethnicity (Diabetes UK, 2014b). Type 2 diabetes is more than six times more common in people of South Asian descent and up to three times more common in those of African–Caribbean descent. These populations also develop type 2 diabetes approximately 10 years earlier than the Caucasian population (Winkley et al, 2013).
Another contributing factor is deprivation, with a higher incidence of both obesity and diabetes identified in lower socioeconomic groups. Marmot et al (2010) emphasised that the conditions we are born, live and work in have a significant impact on our long-term health outcomes. In the UK, the most disadvantaged areas are 2.5 times more likely to develop prediabetes and diabetes than the general population, with women being four times more likely to have the conditions if they are from a poorer economic background than those from the highest income brackets (Diabetes UK, 2012). The prevalence of prediabetes/diabetes is also reportedly two to three times higher in people with severe mental illness compared with the general population (Holt et al, 2005). Despite their high risk of physical ill health, people with mental health problems have less access to preventative and early interventions for physical illness, and may also suffer discrimination within healthcare systems (Thornicroft, 2006).
Clinical effects of prediabetes
The main clinical concern with prediabetes is the high risk that the individual will go on to develop type 2 diabetes. However, prediabetes itself is associated with a number of negative health outcomes.
Bansal (2015) discussed several studies linking prediabetes with increased risk of chronic kidney disease, early-stage nephropathy, neuropathy, retinopathy and macrovascular disease. The US Diabetes Prevention Program (DPP) study showed that almost 8% of those diagnosed with prediabetes had some degree of retinopathy (DPP Research Group, 2007). Other research suggested a link with various neuropathies, ranging from erectile dysfunction to sensory neuropathy (Sumner et al, 2003). A meta-analysis which included studies with a combined participant population of 760925 people also found that a diagnosis of prediabetes increases the risk of stroke by as much as 21% (Lee et al, 2012).
Management of prediabetes
NICE (2012) recommends using an appropriate risk assessment tool, such as the one provided by Diabetes UK (2017), to calculate the overall risk of developing type 2 diabetes. In those identified as high-risk, lifestyle interventions alone can reduce the risk of developing type 2 diabetes by as much as 25–72% (Perreault et al, 2012).
In England, the Healthier You: NHS Diabetes Prevention Programme is currently being operated in 20 pilot areas. Patients at high risk of developing type 2 diabetes can be referred to this programme, where they will be offered personalised support to reduce their risk level. The programme includes healthy eating and lifestyle education, weight loss support and bespoke physical exercise programmes. Studies have shown that a weight loss of just 7% could reduce the risk of developing type 2 diabetes by as much as 58% (ADA, 2014). Guidance provided by NICE (2012) recommends offering these interventions and then reassessing weight and BMI regularly, in addition to offering a repeat blood test yearly. This recommendation is supported by the ADA (2014), which recommends that people identified as having prediabetes undergo further diabetes screens every 1–2 years. Early identification of type 2 diabetes is important, given that as many as 50% of all people recently diagnosed will already have some diabetes-related complications (Diabetes UK, 2015), and the evidence suggests that early interventions can delay or prevent these complications and risks.
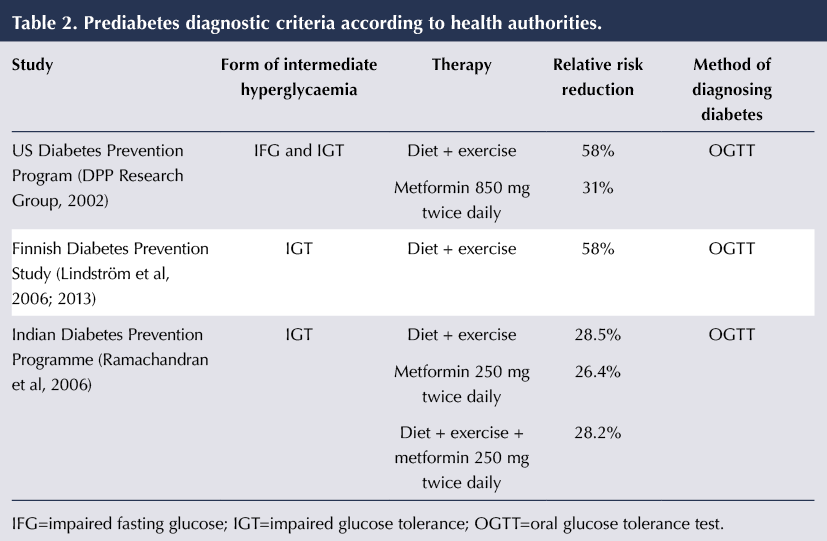
Table 2 summarises the relative risk reductions for the onset of type 2 diabetes in the national diabetes prevention studies conducted in Finland, India and US. These studies support the use of both lifestyle modification and pharmacotherapy as a means to delay or prevent type 2 diabetes in high-risk individuals. The combination of diet and exercise has been shown to reduce diabetes risk by as much as 58% in those with IGT, with similar outcomes noted in the Finnish and US programmes (DPP Research Group, 2002; Lindström et al, 2006). Interestingly, the US DPP concluded that lifestyle intervention was more effective at risk reduction than metformin. Bansal (2015) concluded from this that the initial treatment of choice should be diet and lifestyle intervention, and not medications.
However, individual circumstances should be taken into account, and NICE (2012) allows the prescribing of metformin or orlistat in those people who, despite diet and lifestyle interventions, are still at high risk, and in those who are prevented from fully accessing the intervention, such as those with some disabilities or mental health diagnoses. NICE (2015) stresses the importance of an individualised approach to tackling diabetes by tailoring care to the needs and circumstances of each individual. Recommendations include a person’s preference, comorbidities, polypharmacy, cultural beliefs and ability to benefit from long-term interventions.
Importantly, no significant difference was observed between metformin alone, lifestyle intervention alone and lifestyle intervention plus metformin in the DPP India study, which was conducted solely in an Asian population. This population group is known to have a higher background incidence of diabetes, and the cohort had a lower BMI at start of the study and did not show a reduction in weight during the study period (Ramachandran et al, 2006).
Conclusions
Despite some variation in diagnostic criteria, there is unanimous agreement that prediabetes, borderline diabetes, IFG and IGT pose a serious risk to public health outcomes, morbidity and mortality. However, studies have proven the success of lifestyle change in managing prediabetes and preventing and delaying type 2 diabetes. NICE guidelines allow the prescribing of metformin or orlistat in those people who, despite diet and lifestyle interventions, are still at high risk, although these medications have proved less beneficial than diet and lifestyle in studies. The next challenges are overcoming the barriers to change and addressing socioeconomic inequalities, which will then enable the interventions to be more successful.





Diabetes UK releases its 5-year strategy to tackle diabetes inequities.
16 Jul 2025