Publisher’s note: The advice in this consensus is now out of date. Please see updated joint guidance from PCDS and ABCD, available here.
The PDF of this publication remains online for archival purposes. Please refer to the current version for up-to-date recommendations.
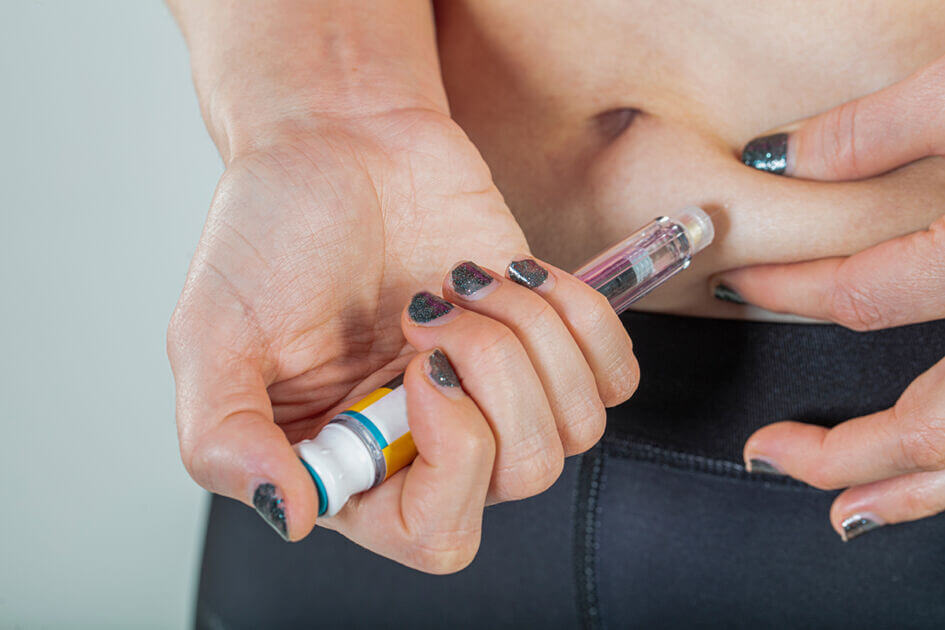





Helping homeless adults to overcome the challenges of managing their condition.
16 Apr 2024