Cystic fibrosis diabetes (CFD) is a common comorbidity in cystic fibrosis (CF). It contributes to a deterioration in clinical status that can begin 2–4 years prior to diagnosis (Goff et al, 2016; Moran et al, 2018). CF is an inherited genetic condition that causes the cystic fibrosis transmembrane conductance regulator (CFTR) protein to change, resulting in thick, viscous, dehydrated secretions in sweat glands and in respiratory, gastrointestinal and reproductive tracts (Ode et al, 2022). In the UK, CF affects over 10800 people and is the most common life-limiting genetic condition (Cystic Fibrosis Trust, 2022a). Features include pulmonary exacerbations, lung failure, pancreatic insufficiency, intestinal obstruction, liver disease and CFD.
Epidemiology
In 2020–21, CFD accounted for 0.6% (192) of children and young people (CYP) with diabetes aged 0–24 years in England and Wales (National Paediatric Diabetes Audit, 2022). The overall figure is likely to be higher, as those aged <24 years who had already transitioned to adult services were not included.
The UK CF Registry annual report for 2021 showed that 29.8% of those with CF aged ≥10 years and 8.3% of those aged 10–15 years required treatment for CFD (CF Trust, 2022a). Again, these figures may have been higher as annual screening for CFD commences from 10 years in the UK while we know that glucose abnormalities can occur earlier, although incidence usually increases with age (Grandados et al, 2019; Kaminski et al, 2019).
Increased incidence of CFD has been found in those with pancreatic insufficiency, poor lung function, steroid use, CF-related liver disease, continuous enteral feeding, females, family history of type 2 diabetes, transplantation, early age glucose abnormality, incretin changes and with certain gene variants (Moran et al, 2018; Granados et al, 2019).
Pathophysiology
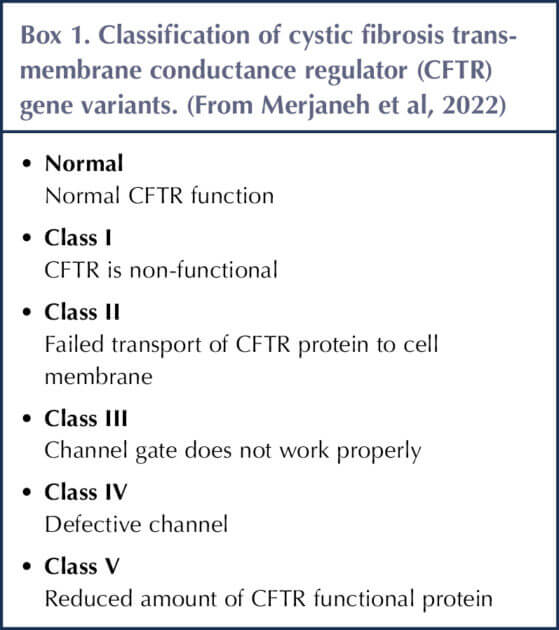
Box 1 shows the different variant classes of CF. Classes I, II and III are associated with higher incidence of CFD (Merjaneh et al, 2022).
CFD differs from type 1 or type 2 diabetes in its management and diagnosis (Ode et al, 2022). In CFD, insulin deficiency occurs because of obstructive damage of islet cells in the pancreas, leading to beta-, alpha- and polypeptide cell loss (Moran et al, 2018). This means that insulin, glucagon and polypeptide secretion is reduced, but not totally lost (Moran et al, 2018).
Reduced insulin secretion results in a catabolic state, leading to deterioration in nutritional status (such as weight loss, reduced BMI and muscle loss) and lung function (such as impaired lung clearance, impaired neutrophils and increased inflammation), resulting in increased morbidity and mortality in CFD (Kaminski et al, 2019; Piona et al, 2022).
Insulin resistance is also seen in CFD, causing hyperglycaemia that can worsen in acute pulmonary exacerbation, chronic lung disease, steroid use or high carbohydrate intake (Perrem et al, 2019). With the introduction of CFTR modulator therapy, insulin resistance may increase in those becoming overweight (Ode et al, 2022). Few people with CF have truly normal glucose tolerance; those without CFD experience insulin resistance and the majority with pancreatic insufficiency will have some beta-cell damage (Moran et al, 2018).
Screening and diagnosis
People with CFD are usually asymptomatic, but may experience polyuria, polydipsia, the inability to gain/maintain weight despite intervention, poor growth velocity, delayed puberty or unexplained reduced lung function (Moran et al, 2018; Frost et al, 2019). Incidence of diabetic ketoacidosis is low, owing to incomplete loss of insulin and reduced glucagon production, but it is important to rule out type 1 diabetes, as cases have been reported (Ode et al, 2022).
Annual CFD screening from 10 years old ensures early detection and prevents poor outcomes associated with CFD. If symptomatic, CFD should be screened for immediately. While Ode et al (2022) found CFD to be rare in children <10 years old, they also noted that some centres begin screening at 6 years old, as one American retrospective study reported that 6–9-year-olds in whom glucose abnormalities were detected were at an increased risk of developing CFD in early adolescence (Ode et al, 2010). Screening before 6 years of age proved difficult for practical and logistical reasons. It is important to note that this study was carried out before modulator therapy was introduced, which may now impact glucose metabolism.
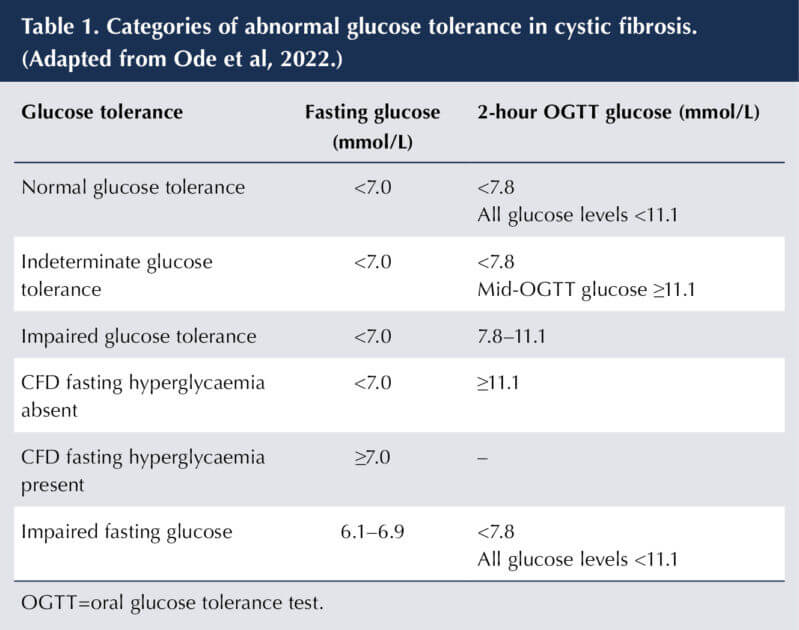
Current oral glucose tolerance test (OGTT) screening using 1.75 g glucose/kg (maximum 75 g) is recommended, as there is historical data linking results to outcomes such as lung function and/or weight deterioration (Ode et al, 2022). Categories of abnormal glucose tolerance are shown in Table 1.
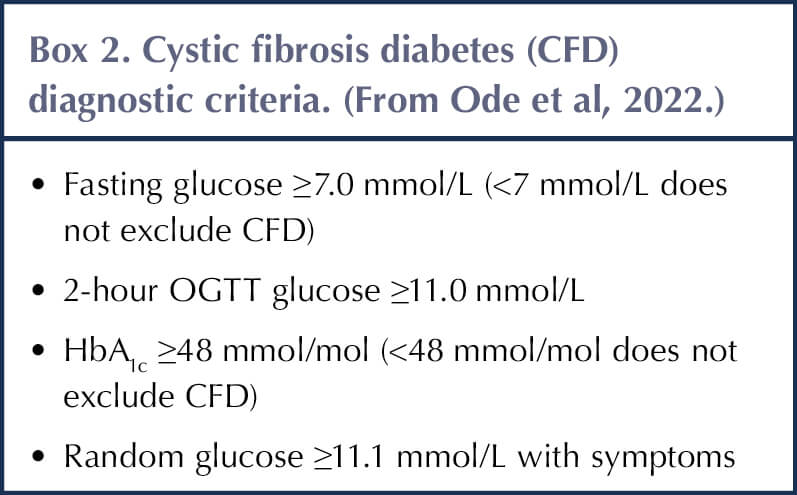
Ode et al (2022) recommend that during stable health CFD can also be diagnosed using criteria seen in Box 2. A diagnosis can be made during acute illness if fasting glucose is ≥7.0 mmol/l or 2-hour postprandial glucose is ≥11.1 mmol/L persist for over 48 hours.
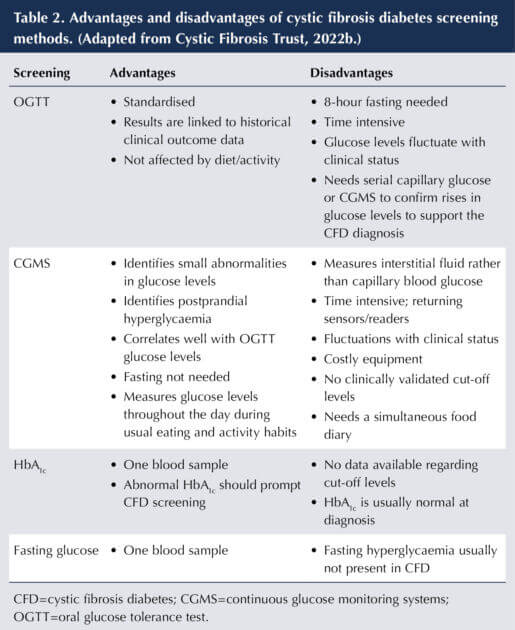
Variability exists in CFD screening practices, with some using continuous glucose monitoring systems (CGMS; Perrem et al, 2019). Table 2 provides a comparison of screening methods. Screening with CGMS allows for the early identification of glucose abnormalities even if OGTT levels are normal, and enables earlier intervention. Furthermore, studies report a connection between CGMS abnormalities and clinical deterioration (Southern et al, 2022). In contrast, Ode et al (2022) suggest that CGMS is not suitable for diagnosis and screening currently, owing to lack of guidance on interpreting data.
Management
CFD should be treated with insulin, and treatment discussed with the paediatric diabetes team. Doses are usually lower than in type 1 diabetes. Oral antidiabetes agents are not currently recommended in CFD owing to a limited number of studies, including since modulator therapy was introduced (Ode et al, 2022).
Insulin improves nutritional status and lung function, reducing mortality and pulmonary exacerbations (Granados et al, 2019). Individualised education, which includes injection technique, site rotation, hypoglycaemia treatment and exercise management, is led by the diabetes team. Face-to-face group education sessions would not be advisable in order to prevent cross infection (Frost et al, 2019).
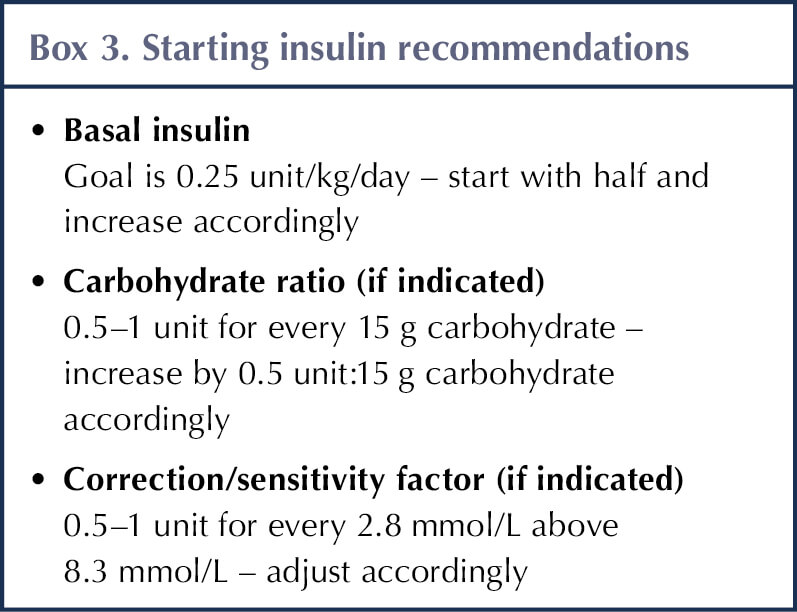
Insulin regimens are individualised, and some individuals without fasting hyperglycaemia may only need basal or bolus insulin injections (Moran et al, 2018; Ode et al, 2019). For those requiring basal and bolus insulin (fasting hyperglycaemia), carbohydrate-counting education is recommended, with starting doses of <0.5–0.8 units/kg/day in a typical state of health (Kaminski et al, 2019; Ode et al, 2022). A summary of recommendations is provided in Box 3.
For those requiring basal and bolus insulin, insulin pump therapy should be considered, including closed-loop therapies (Ode et al, 2022). Benefits include fewer injections, the possibility of more frequent snacking and improved blood glucose control.
Monitoring of glucose levels is important. Once a day may be adequate for basal insulin only, but those on basal and bolus insulins or pump therapy should be monitored more frequently. Use of CGMS is encouraged in established CFD to help with insulin adjustment and alerting hypoglycaemia. Such systems provides teams with remote access to data to enable close monitoring and insulin adjustment (Frost et al, 2019).
There are currently no defined glycaemia targets for CFD. NICE (2022) guidance for CYP with type 1 diabetes recommends an HbA1c ≤48 mmol/mol, a blood glucose level of 5–7 mmol/L fasting/on waking, 4–7 mmol/L pre meal and 5–9 mmol/L post meal. There are also no CGMS target guidelines for CFD, but Chan et al (2019) recommends trying to achieve those set for pregnancy (3.5–7.8 mmol/L over 70% of the time and >7.8 mmol/L less than 25%), while minimising hypos (<3.5 mmol/L less than 4% and <3.0 mmol/L less than 1%; Battelino et al, 2019).
Acceptance of advanced diabetes technologies, including insulin pumps without or with CGMS in an open, partial or hybrid closed-loop form, can be low in CFD (Chan et al, 2019). Although specific CFD algorithms are not currently available and evidence supporting use in CFD is limited, such devices would be beneficial in improving quality of life.
Nutritional intervention is individualised and may change with introduction of modulator therapy. A high calorie, salt, protein and fat diet should be continued without carbohydrate restriction to support growth and fibre intake (Frost et al, 2019; Kaminski et al, 2019; Ode et al, 2019). Adequate pancreatic enzyme dosing contributes to improving glucose control and nutritional status (Frost et al, 2019; Kaminski et al, 2019). Furthermore, Moran et al (2018) recommend moderate aerobic activity for at least 150 minutes per week to help improve glucose control.
Modulator therapy (modulators)
Introduction of CFTR modulators has improved sweat chloride levels, lung function, pulmonary exacerbation and nutritional status, thereby reducing mortality (Crow et al, 2022). Modulators target the defective CFTR protein in certain gene variants, improving or restoring CFTR protein function. Some CFTR gene variants are not responsive to modulators (Southern et al, 2022). Currently there are four modulator types available: ivacaftor, lumacaftor–ivacaftor, tezacaftor–ivacaftor and elexacaftor–tezacaftor–ivacaftor (triple therapy). Impact on blood glucose in CFD requires further research, as results have been mixed and limited. Glucose levels should, therefore, be monitored closely after starting modulators and insulin adjusted accordingly (Piona et al, 2022).
There is evidence suggesting CFTR channels exist in beta-cells, and defects in CFTR have been found to contribute to insulin production abnormalities, but it is unknown whether modulators may improve glucose metabolism (Li et al, 2019). In addition, modulators have been found to affect glucose handling, but further studies are needed (Southern et al, 2022). Furthermore, Merjaneh et al (2022) have suggested possible mechanisms by which triple therapy and ivacaftor may affect glucose metabolism. These include weight gain increasing insulin resistance; reduced inflammation and increased activity increasing insulin sensitivity; and the effect on islet cells improving insulin secretion. With life expectancy increasing, incidence of macrovascular complications may become more common and may need monitoring alongside annual microvascular screening (Frost et al, 2019).
Interestingly, it has been hypothesised from Canadian data that CFD incidence may be reducing in line with improved pulmonary status owing to better treatments, but further studies would be beneficial as CYP screened were not taking modulators (Perrem et al, 2019).
Multidisciplinary team working
Ideally, management of CFD is conducted by the multidisciplinary team. Joint CF and diabetes team clinics every 3 months are recommended to help optimise care (Frost et al, 2019; Ode el al, 2022). An allocated professional to be a link between the teams and the key worker is advisable. This role could be carried out by a dietitian, as they provide essential education on nutrition intervention, or by a specialist CF diabetes nurse (Frost et al, 2019; Kaminski et al, 2019). This key worker could support the young person and act as their first point of contact. They could also be the coordinator/link between teams, lead on transition to adult care, support education providers and keep teams up to date on changes in CFD care and how CFD differs from other types of diabetes (Frost et al, 2019).
The Best Practice Tariff for paediatric diabetes requires that all CYP, including those with CFD, have access to a diabetes psychologist. The impact of an additional diagnosis is a significant burden for CYP, and is likely to impact on their mental health (Frost et al, 2019; Kaminski et al, 2019; Li et al, 2019). Typically, diagnosis of CFD can occur in parallel with other life transitional and developmental stages, which put further strain on a young person with CF (Frost et al, 2019). Psychological support is limited at some centres, but a key worker may be able to contribute with support.
Conclusion
Early identification of CFD and commencement of insulin contribute significantly towards improving health outcomes in CYP with CF. Screening of CFD from 6 years of age would allow centres to identify those with early glucose abnormalities and initiate early intervention, whether monitoring or treatment. More research is needed into early treatment when glucose abnormalities are first detected, and greater guidance on using CGMS in screening is required. For those with established CFD, official guidance on optimal glycaemic targets would be beneficial. Further research to support the use of advanced diabetes technologies in CFD, including hybrid closed-loop insulin pumps, is required, along with a greater understanding of the impact of modulator treatment on CFD.
Acknowledgement
The author would like to thank the Children and Young People’s Diabetes Care Module, Birmingham City University, for its support in the writing of this article.





Study provides new clues to why this condition is more aggressive in young children.
14 Nov 2025