Recent figures estimate that approximately 4.6 million people in the UK are living with diabetes. Of those with a diagnosis, around 8% have type 1 diabetes and 90% have type 2 diabetes, while other forms of diabetes make up the remaining 2% (Diabetes UK, 2025). These rarer forms of diabetes have been grouped into nine categories by the International Diabetes Federation (2025; see Table 1). This article will focus on maturity-onset diabetes of the young (MODY) by reviewing its different types and providing some advice on diagnosing and managing the condition.
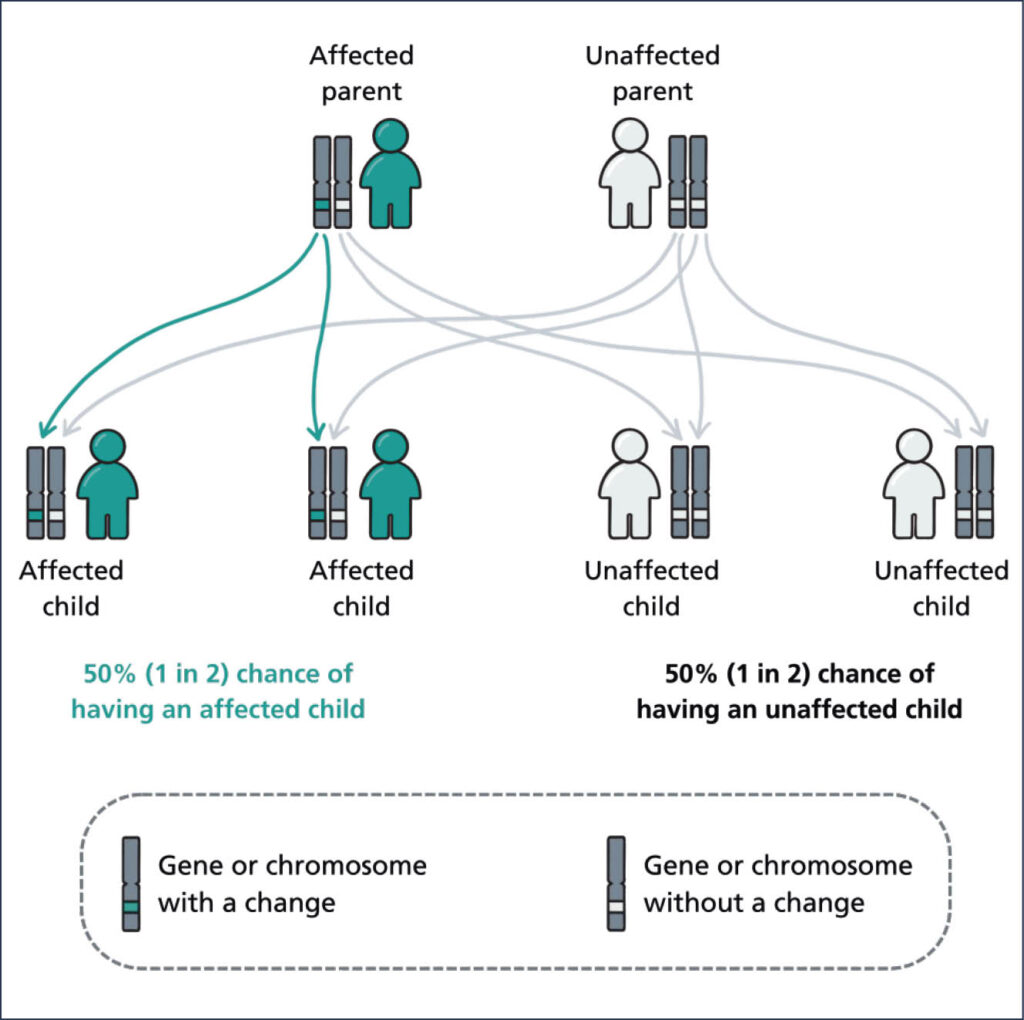
MODY is inherited when a single gene mutation is passed by an affected parent to their child in an autosomal dominant pattern (Diabetes Genes, 2025; Figure 1). This means that a child with one affected parent has a 50% chance of inheriting the gene and, therefore, being affected. The gene mutation can lead to impaired insulin secretion or beta-cell function, resulting in elevated blood glucose levels (Sami et al, 2025). Individuals presenting with MODY are often misclassified as having type 1 and type 2 diabetes owing to a lack of awareness in healthcare professionals and overlapping symptoms. It can take many years for a definitive genetic diagnosis to be received (Genomics Education Programme, 2019).
The Diabetes Genes website, created by the University of Exeter, provides in-depth information about MODY. It lists the three main features of MODY as:
- Diabetes often developing before the age of 25 years.
- Diabetes that runs in families from one generation to the next.
- Diabetes that may be treated by diet or tablets and does not always need insulin treatment.
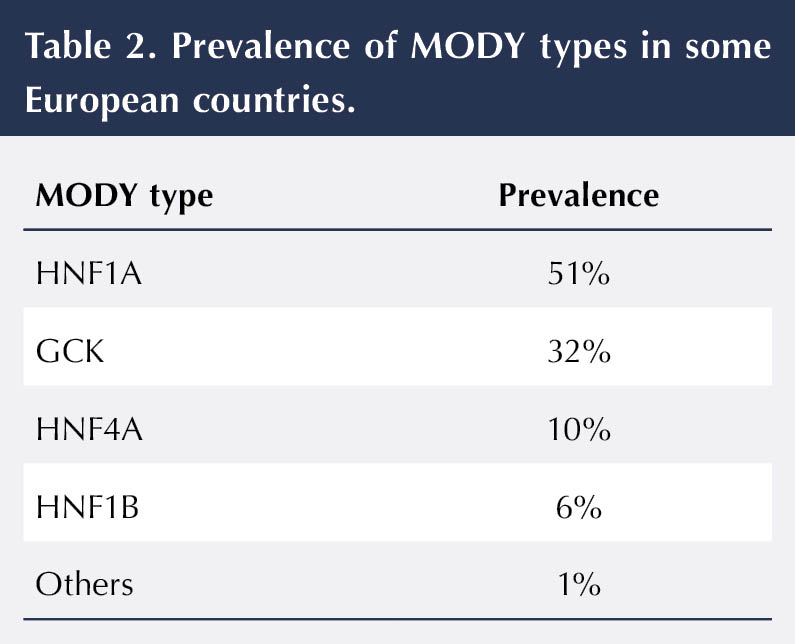
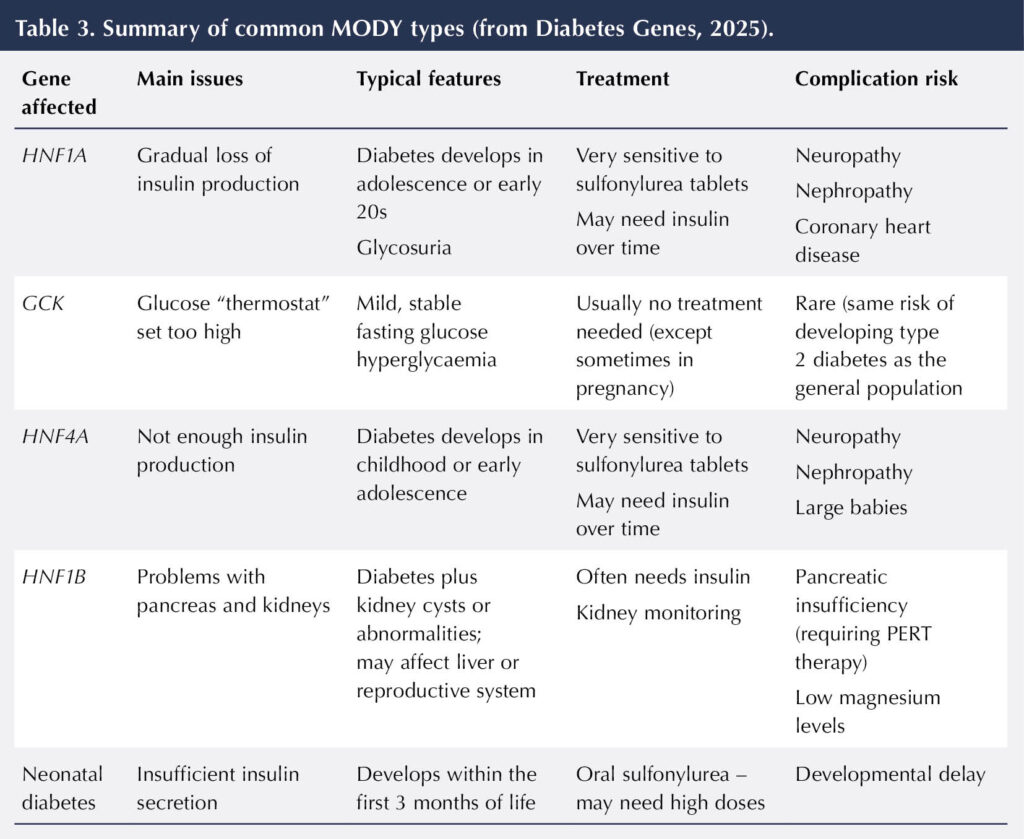
As there are different types of MODY, it is important that an individual receives the correct diagnosis so that the most appropriate treatment option can be determined. It will also help healthcare professionals to inform people how their diabetes will progress over time (Diabetes Genes, 2025). Mutations in different genes lead to these different MODY types (Table 2 and Table 3) and are reviewed below.
HNF1A
HNF1A is the most common form of MODY. Changes in the HNF1A gene reduce the amount of insulin produced by the pancreas, which results in diabetes. In childhood, insulin production is usually normal, but this reduces with age, so diabetes usually develops in adolescence or the early twenties.
Individuals with HNF1A are prone to complications, such as neuropathy and nephropathy, as well as an increased risk of coronary heart disease. Individuals often have a family history of a myocardial infarction at an early age and require statin therapy at least from the age of 40 years. They may also have glucose in their urine, despite having glucose levels within a normal range.
Treatment for HNF1A is with a sulfonylurea medication (such as gliclazide), often at low doses as individuals can be extremely sensitive to them (Shepherd et al, 2009). Individuals who are taking insulin prior to their diagnosis of HNF1A may be able to switch to a sulfonylurea, and achieve improved glucose control and reduced risk of hypoglycaemia (Shepherd et al, 2009).
In summary:
- Progressive beta-cell defect.
- Presents in adolescence/young adulthood.
- Very sensitive to sulfonylureas.
Glucokinase (GCK)
The glucokinase (GCK) gene plays a role in how high blood glucose is within the body. It codes for the glucokinase enzyme, which helps to maintain glucose levels by acting on the pancreas to produce more insulin if levels rise. Changes to the GCK gene can result in slightly elevated glucose levels, typically between 5.5 and 8.0 mmol/L (compared to a level of around 5.5 mmol/L in an individual without this change).
There are often no physical symptoms and, if medication is started, it has little effect on the glucose levels, as the body will try and reset to the slightly raised level.
GCK is often picked up incidentally from a random blood glucose test, particularly in children. In pregnancy, it is often also misdiagnosed as gestational diabetes, which is why a review of an individual’s family history of diabetes is so important. If a pregnant woman has GCK, the baby may grow more quickly. On occasions, insulin is used to help control the baby’s growth.
In summary:
- Easiest to detect in young people, including children (consider when present with incidental hyperglycaemia with FBG >5.5 mmol/L on two occasions).
- Often misdiagnosed as gestational diabetes (commonest presentation in the UK).
- Usually, no treatment is required.
HNF4A
Changes in the HNF4A gene can cause diabetes by reducing the amount of insulin the pancreas can produce. As with HNF1A, insulin production reduces with age, so individuals typically develop diabetes in childhood or adolescence. Babies who inherit HNF4A can be macrosomic at birth (>4 kg) and may suffer with neonatal hypoglycaemia. As with HNF1A, the treatment in HNF4A is sulfonylurea medication, which stimulates the pancreas to produce more insulin (Diabetes Genes, 2025).
In summary:
- Progressive beta-cell defect.
- Presents in adolescence/young adulthood.
- Sensitive to sulfonylureas.
- Macrosomia ≥4 kg (owing to over-secretion of insulin).
- With or without hypoglycaemia at birth and for the first few weeks/months.
HNF1B
The HNF1B gene provides instructions to make a protein that is found in many organs, such as the liver, lungs, pancreas, kidneys and reproductive system. This form of MODY is slightly more complex, and is often referred to as renal cysts and diabetes (RCAD), as the faulty gene can lead to structural problems, such as cysts or having a single kidney. Individuals may also have abnormalities within their urinary tract. The diabetes in individuals with HNF1B commonly develops after the kidney disease, and usually requires insulin. There is an increased incidence of neurodevelopmental disorders, such as autism, within individuals with HNF1B (The European Rare Kidney Disease Reference Network, 2025).
In summary:
- Renal ultrasound – multiple cysts in kidneys, renal function normal (renal abnormalities may be seen in utero).
- Faecal elastase reduced, indicating moderate pancreatic insufficiency.
- Low magnesium levels.
- Diabetes develops after the renal disease (usually requires insulin).
- Autism often seen in individuals with whole gene deletion of HNF1B.
Neonatal diabetes (ABCC8 or KCNJ11)
Changes to the ABCC8 or KCNJ11 genes can cause transient neonatal diabetes (TNDM) or permanent neonatal diabetes (PNDM) by disrupting the secretion of insulin in response to glucose levels. These are usually identified by diabetes developing in the first weeks of life, with babies often presenting in diabetic ketoacidosis (DKA). Many individuals are misdiagnosed as having type 1 diabetes owing to the presentation.
With TNDM, there is usually a period of remission between the ages of one and 15 years, followed by relapse. Individuals with TNDM usually have significant endogenous insulin production with measurable C-peptide levels (Diabetes Genes, 2025). A sulfonylurea, such as glibenclamide, is usually the treatment of choice. Around 90% of individuals with PNDM can switch successfully from insulin therapy to a sulfonylurea (again, glibenclamide is recommended, but typically at higher doses than used with type 2 diabetes; Diabetes Genes, 2025).
In summary:
- Diabetes diagnosed before the age of 6 months.
- Sensitive to high dose of sulfonylurea.
- Around 90% individuals with PNDM can discontinue insulin.
What to do if you think you have an individual why may have MODY
1. Check family history
When considering MODY, it is important to collate the diabetes family history of that individual. It often helps to draw this out in a family tree. NHS England’s Genomics Education Programme (2025) provides free educational resources to help you do this. There is generally a strong inheritance pattern with families with MODY (autosomal dominance). The key details to gather for each affected family member are:
- Age at diagnosis (often before 25 years).
- Body habitus at diagnosis (often not overweight/obese, unlike typical type 2 diabetes).
- Insulin use (many individuals with MODY manage without insulin for years; Hoffman et al, 2023).
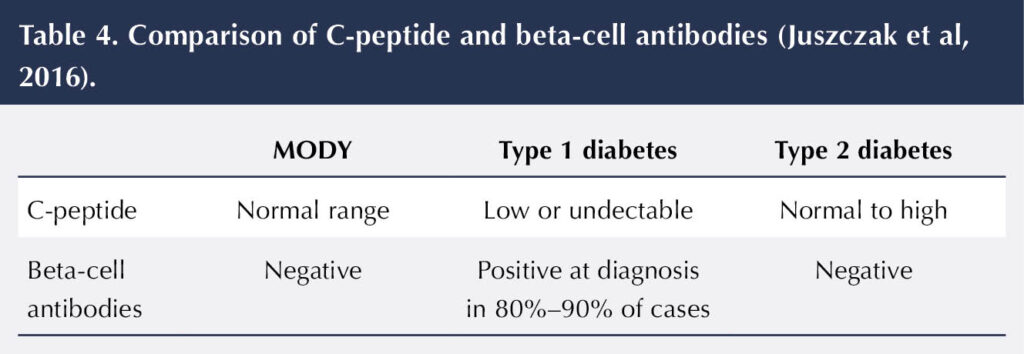
2. Check C-peptide and beta-cell antibody levels
C-peptide level is a measure of how much insulin an individual is producing themselves (Diabetes Genes, 2025). A low level is more suggestive of type 1 diabetes. The level of C-peptide falls over time and is usually undetectable in individuals with type 1 diabetes in the first 1–3 years post diagnosis (Juszczak et al, 2016). In comparison, elevated levels of C-peptide are more typical in individuals with type 2 diabetes.
Testing for beta-cell antibodies is another useful way to help discriminate between diabetes types. These antibodies are indicators of the autoimmune process that is associated with type 1 diabetes (Diabetes Genes, 2025). There are three beta-cell antibodies that can be tested for (GAD, IA2 and ZnT8). Each remains detectable for a varying time after diagnosis of type 1 diabetes. Table 4 shows a comparison of how C-peptide and beta-cell antibodies help with the diagnosis of MODY.
3. Mody calculator (www.diabetesgenes.org)
The University of Exeter has developed an online MODY probability calculator that provides a probability score for an individual having MODY. They have also developed a free Diabetes Diagnostics App that can indicate the most likely type of diabetes and the likelihood of the individual having MODY. Both these resources require a few clinical details to be entered, such as age at diagnosis, HbA1c, BMI and whether a parent of the individual has diabetes.
4. Genetic testing
The NHS England National Genomic Test Directory: Testing Criteria for Rare and Inherited Disease was first published in October 2021. It sets out the criteria for testing of MODY, as the cost for genetic testing is now centrally funded by the NHS (NHS England, 2024). The criteria are presented below:
Patients with isolated diabetes should be tested if they have:
a. Diabetes diagnosed young (≤35 years in White Europeans and ≤30 years in high prevalence ethnic groups like South Asians)
AND
b. Unlikely to have type 1 diabetes because:
They are not on insulin treatment
OR
They are on insulin treatment with all autoantibodies tested negative (minimum testing of GADA and IA2A) and a random non-fasting C-peptide value ≥200 pmol
AND
c. Have features suggestive of MODY:
An HbA1c at diagnosis of diabetes <58 mmol/mol (<7.5%), if diagnosed under 18 years of age,
OR
BMI <30 kg/m2 adult (child BMI <95th centile) and a parent with diabetes (if White) or BMI <27 kg/m2 (child BMI <95th centile) and a parent with diabetes (if high prevalence type 2 diabetes ethnic group)
OR
Have a MODY probability score ≥20% if not insulin treated and ≥10% if insulin treated (see the MODY calculator).
With regards to GCK genetic testing, individuals should be tested if they have:
a. Fasting glucose noted to be raised ≤35 years
AND
b. Asymptomatic stable fasting hyperglycaemia (5.5–8 mmol/L) (minimum two independent laboratory fasting blood glucose test results)
OR
c. HbA1c 36–58 mmol/mol (5.5%–7.5%).
Testing criteria in pregnancy
a. Gestational diabetes with fasting glucose 5.5–8 mmol/L
AND
b. BMI <30 kg/m2 if White, or BMI <27 kg/m2, if high prevalence type 2 diabetes ethnic group.
For neonatal diabetes
Any individual who was diagnosed under the age of 9 months of age can be tested.





Su Down reflects on the capacity for advances in diabetes care to keep surprising her.
15 Oct 2025