The glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are powerful glucose-lowering agents in the context of type 2 diabetes (T2D). They also offer the possibility of weight loss for overweight/obese people with or without T2D. This article summarises the position reached with the GLP-1 RAs and reviews the developing field of unimolecular co-agonists that act not only on the GLP-1 receptor, but on other receptors, notably glucose-dependent insulotropic polypeptide (GIP) and glucagon receptors, with the aim of further improving glycaemic control and weight reduction.
Incretin hormones – what they are and what they do
The incretin hormones are secreted from the small intestine in response to oral intake of food and, in particular, the presence of glucose in the gut. There are two principal incretin agents: GIP and GLP-1. GIP and GLP-1 are polypeptides that are degraded in vivo within minutes by hydrolysis of peptide bonds catalysed by the enzyme dipeptidyl-peptidase 4 (DPP-4; Drucker and Nauk, 2006).
GIP and GLP-1 stimulate insulin release by binding to GIP and GLP-1 receptors, respectively, in pancreatic beta cells. This insulotropic activity lowers blood glucose levels, reducing the peak of post-prandial hyperglycaemia. Importantly, the activation of these hormones is glucose-dependent, operating in situations of hyperglycaemia.
GLP-1 may further counter post-prandial hyperglycaemia by suppressing glucagon release from the alpha cells of the pancreas, in the context of hyper- or euglycaemia. Importantly, GLP-1 loses its inhibitory effect on glucagon secretion when blood glucose levels fall, allowing an appropriate glucagon response to hypoglycaemia. In contrast, GIP positively stimulates glucagon secretion at lower levels of glycaemia (Seino et al, 2010).
In addition, the incretin hormones slow gastric emptying, thereby delaying the absorption of food (and, in particular, glucose) – a further mechanism in reducing post-meal hyperglycaemia. They also induce satiety, reducing appetite and calorie intake, thus enabling weight loss via a direct effect on the hypothalamus (Nauck and Meier, 2016).
Incretin hormones and type 2 diabetes
In people without T2D, GIP is the dominant incretin hormone, having a greater insulotropic effect than GLP-1 (Nauck and Meier, 2016). This situation is reversed in people with T2D where, although GIP secretion remains largely intact, GIP receptor activity is much reduced. Whilst declining beta-cell function is thought to generally reduce the response to incretins, GLP-1 appeared to be the more important incretin hormone in T2D (Nauck et al, 2021).
Thus, GLP-1 RAs, rather than GIP RAs, were initially identified as a key therapeutic target.
The development of GLP-1 receptor agonists
Native GLP-1 is unsuitable itself as a therapeutic agent because of its short half-life in vivo. The strategy was, therefore, to identify GLP-1 mimetic drugs with a molecular structure close enough to native GLP-1 to stimulate the GLP-1 receptor, but with alterations that enabled prolonged activity by, in particular, possessing resistance to breakdown by DPP-4 (Meier, 2012).
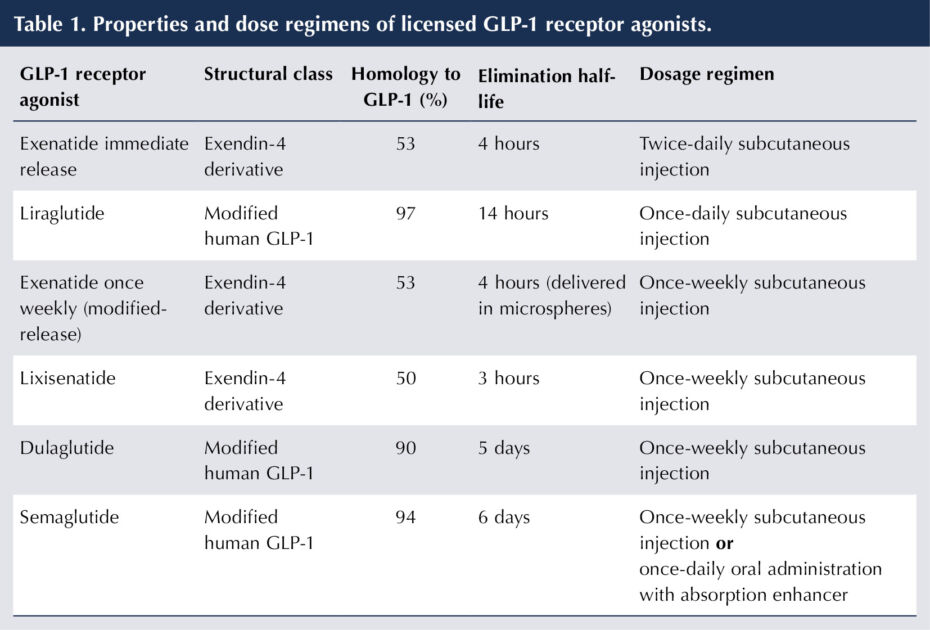
In fact, the GLP-1 RAs can be categorised structurally into two major groups (see Table 1; Romera et al, 2019; Nauck et al, 2021). The first comprises the exendin-4 derivatives (exenatide and lixisenatide), whose structure is based on the peptide sequence of exendin-4, which was isolated from the saliva of the Gila monster lizard (Nauck et al, 2021).
The second group (liraglutide, dulaglutide and semaglutide) have a modified human GLP-1 structure and, hence, a much closer homology to that of GLP-1 itself.
Alongside GLP-1 RAs, the DPP-4 inhibitors have been developed to exploit the incretin system by prolonging the duration of endogenous GLP-1 (Nauck and Meier, 2016).
The rise of GLP-1 receptor agonists for use in type 2 diabetes
Of the non-insulin treatments available for treating T2D, GLP-1 RAs offer the greatest reductions in HbA1c, with more recent long-acting GLP-1 RAs matching, or even exceeding, glycaemic improvement achieved with basal insulin in T2D (Nauck et al, 2021). Their glucose-dependent mechanism of action means they intrinsically carry a low risk of inducing hypoglycaemia. A further important clinical benefit is weight loss. These factors have led to the ADA/EASD recommendation that GLP-1 RAs be the preferred first injectable glucose-lowering agent for T2D (ahead of insulin therapy; Davies et al, 2018).
A further drive to utilise GLP-1 RAs arises from the cardiorenal benefits offered with some of the therapies. There is strong evidence from randomised cardiovascular outcome trials (CVOTs) that several long-acting GLP-1 RAs (liraglutide, dulaglutide and subcutaneous semaglutide) provide cardiovascular protection to individuals with T2D with pre-existing cardiovascular disease (secondary prevention) or at high risk of CV events, and to a lesser extent those without overt atherosclerotic vascular disease (primary prevention; Marso et al, 2016a; 2016b; Gerstein et al, 2019).
GLP-1 RAs consistently improved composite (secondary) renal outcomes in the CVOTs, principally driven by a reduction in new-onset albuminuria. Thus, they are a logical treatment choice in diabetic nephropathy, after SGLT2 inhibitors. GLP-1 RAs may also have utility in non-alcoholic steatohepatitis (NASH).
Meta-analyses have confirmed the benefit of GLP-1 RAs as a class in both secondary and primary prevention of cardiovascular disease, and in reduction of adverse kidney outcomes in people with T2D (Kristiensen et al, 2019; Marsico et al, 2020).
In the context of T2D, weight loss with the GLP-1 RAs reaches a plateau after around 6 months of treatment. It varies between treatments, typically from around 1.5 to 6 kg, the highest reductions being found with semaglutide, both subcutaneous and oral preparations (>liraglutide>dulaglutide> exenatide>lixisenatide; Davies et al, 2018; Husain et al, 2019; Nauck et al, 2019).
The application of GLP-1 RAs to managing obesity
Data suggest that higher doses of GLP-1 RAs can generate greater weight losses. Significant weight loss in obese/overweight people was demonstrated for liraglutide versus placebo in populations with and without T2D in the SCALE trials (Davies et al, 2015; Pi-Sunyer et al, 2015).
The results of selected randomised controlled trials of injectable semaglutide 2.4 mg once weekly versus placebo for weight loss in overweight/obesity are summarised in Table 2.
The injectable GLP-1 RAs liraglutide 3 mg once daily and semaglutide 2.4 mg once weekly have both gained a licence for weight loss, alongside lifestyle management, in obese/overweight people without T2D (SmPC, 2023b; 2023c). Steady dose escalation to higher doses than for glycaemic control are indicated if:
- BMI is ≥30 kg/m2, or
- BMI 27 to <30 kg/m2, if there is at least one weight-related comorbidity (including prediabetes and T2D, hypertension, hyperlipidaemia or obstructive sleep apnoea).
Liraglutide 3 mg once daily (Saxenda) has been recommended by NICE (alongside reduced-calorie diet and increased physical activity) for individuals with non-diabetic hyperglycaemia (impaired glucose regulation or “prediabetes” – defined as an HbA1c 42–47 mmol/mol or fasting glucose 5.5–6.9 mmol/L), having a BMI of ≥35 kg/m2 (or ≥32.5 kg/m2 in a higher-risk ethnic minority group) and being at high risk of cardiovascular disease (e.g. hypertension or hyperlipidaemia; NICE, 2020; NICE, 2022). The advice is that treatment should be initiated from a tier 3 weight management service.
Semaglutide 2.4 mg once weekly (Wegovy) has been recently recommended by NICE for overweight and obesity in people with at least one weight-related comorbidity up to a maximum duration of 2 years, again within the context of a specialist weight management service (NICE, 2022; NICE, 2023a).

Practicalities of GLP-1 receptor agonist administration
GLP-1 RAs are polypeptides and, thus, the administration route developed was that of subcutaneous injection. Changes in molecular structure to extend half-life have enabled once-weekly preparations to become available (Nauck et al, 2021).
Gastrointestinal symptoms (nausea, vomiting and diarrhoea) are relatively common with GLP-1 RAs (Bettge et al, 2017). Side effects are most pronounced on commencing therapy and tend to gradually resolve with time. They may be minimised by gradual up-titration of dose. Contraindications (which vary slightly between EU and US labels) and reasons to avoid GLP-1 RAs are summarised in Box 1 (Morris, 2020). For people with significant diabetic retinopathy, caution should be exercised in the use of semaglutide, following the finding of worsening retinopathy in the SUSTAIN-6 trial (Marso et al, 2016a). However, rapid reduction of HbA1c does not appear to be associated with progression of mild or moderate non-proliferative retinopathy (Simó et al, 2023). The GLP-1 RAs are a significantly more expensive item than other medications for T2D.

Oral GLP-1 receptor agonists
A further remarkable development has been that of an orally administered GLP-1 RA (Pratley et al, 2019). Conventional wisdom would argue against the feasibility of an oral polypeptide being absorbed intact into the blood stream. However, oral semaglutide, up to an approved dose of 14 mg once daily, is licensed for glucose-lowering in T2D. It is co-administered with salcaprozate sodium (SNAC), an intestinal permeation enhancer that facilitates rapid absorption of semaglutide across the gut wall, enabling a small but reproducible amount of active drug to enter the circulation, provided dosing instructions are followed carefully (Pearson et al, 2019). The CVOT with oral semaglutide is ongoing.
At licensed doses of 7 and 14 mg once daily, oral semaglutide does not achieve as great a reduction in HbA1c (typically 1.0%–1.4% in the PIONEER programme) and body weight as once-weekly injectable semaglutide. Recently in the PIONEER PLUS study, however, higher doses of oral semaglutide have been found to be more effective in reducing HbA1c and body weight in adults with inadequately controlled T2D, although gastrointestinal side effects, which were mostly judged to be mild to moderate, were more frequent (see Table 3; Aroda et al, 2023).
A double-blind randomised controlled trial (DBRCT) in adults with overweight/obesity without T2D demonstrated that oral semaglutide 50 mg once daily led to a superior decrease in mean body weight compared to placebo after 68 weeks (an estimated 12.7% difference; Knopp et al, 2023). Oral semaglutide is not yet licensed for weight loss in the UK.
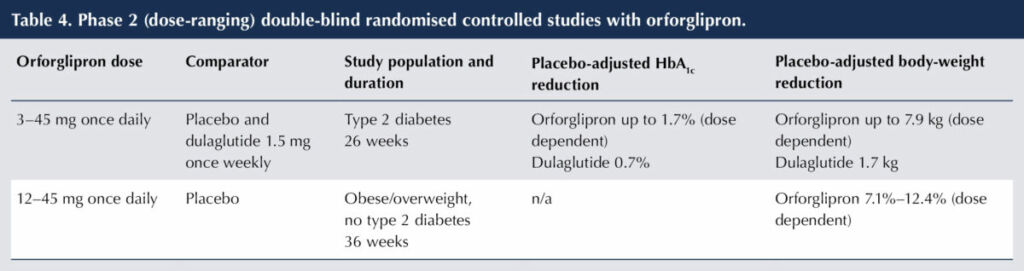
A promising development is orforglipron, a once-daily, non-peptide, oral GLP-1 RA. The efficacy and safety of orforglipron at various doses in people with T2D has been investigated in a phase 2 (dose-ranging) DBRCT versus placebo and dulaglutide 1.5 mg once weekly (Frias et al, 2023) and in a phase 2 DBRCT in a population without T2D to assess weight reduction (Wharton et al, 2023). The results are shown in Table 4.
The adverse effect profile of orforglipron in these trials was similar to that of other GLP-1 RAs. Gastrointestinal side effects were common and exceeded those of dulaglutide in the study of people with T2D, although the majority were classified as mild-to-moderate severity. In the non-diabetes study with orforglipron, there was a dose-dependent discontinuation rate of 10%–17%.
The problem of peptide absorption is thus bypassed with orforglipron, meaning it can be taken without special restrictions or the need for an absorption enhancer. Thus, it might be an attractive alternative to injectable GLP-1 RAs, and more convenient to take than oral semaglutide.

GIP receptor agonism – a useful addition to GLP-1 activity?
An accepted principle has been that resistance to the effects of GIP in people with T2D makes GIP-based treatments unattractive. This line of thought has been challenged following evidence that resistance to GIP can be countered by improvements in glycaemic control from use of antidiabetes medication, thus restoring the insulinotropic potential of GIP in those with T2D (Hojberg et al, 2009). So, using a GLP-1 RA to control glucose levels could simultaneously facilitate the action of a GIP RA.
It has been verified that GIP stabilises blood glucose levels in people with T2D by counteracting both hypoglycaemia (through glucagon secretion) and hyperglycaemia (through insulin secretion; Christensen et al, 2014). There is evidence that GLP-1 and GIP activate different centres in the hypothalamus, permitting greater appetite suppression, and hence weight loss, than either hormone alone (Adriaenssenes et al, 2019).
Such observations led to interest in developing GIP RA therapy in combination with GLP-1 RAs, as a means of amplifying glucose-lowering activity and appetite suppressing activity (Baggio and Drucker, 2021).
Tirzepatide – a combined GIP and GLP-1 receptor agonist
The most clinically advanced unimolecular GIP/GLP-1 RA (so called “twincretin”) is tirzepatide. Its 39 amino acid backbone is based on GIP, with substitution of the second amino acid to avoid degradation with DPP-4, and addition of a C20 fatty acid side-chain that enables binding to albumin. This prolongs half-life to around 5 days, to allow once-weekly administration by subcutaneous injection (Coskun et al, 2018). Tirzepatide displays similar potency to endogenous GIP at the GIP receptor site, though is less potent than native GLP-1 at the GLP-1 receptor site.
Tirzepatide – trials in type 2 diabetes
The SURPASS randomised controlled trials looked at tirzepatide use in individuals with T2D, with the primary outcome being change in HbA1c, and change in body weight a secondary outcome.
SURPASS-1 (Table 5; Rosenstock et al, 2021) demonstrated that tirzepatide could achieve impressive improvements in glycaemic control coupled with substantial weight loss in subjects with T2D. Discontinuation rates ran at a 4.3%–7.1% (dose dependent) in the tirzepatide groups versus 2.6% with placebo.
The most potent GLP-1 RA in clinical practice appears to be once-weekly, subcutaneously injected semaglutide, generating HbA1c reduction up to 20 mmol/mol (1.8%) and weight loss up to 6.5 kg (Pratley et al, 2018). The SURPASS-2 trial (see Table 5; Frias et al, 2021) established tirzepatide as a more potent agent for glycaemic control in subjects with T2D than semaglutide 1 mg once weekly, and as a secondary endpoint indicated that superior weight loss could be achieved.
As expected, gastrointestinal side effects – nausea, vomiting and diarrhoea – were seen in the SURPASS trials, although these were classed as mild to moderate and transient in nature. There was no significant hypoglycaemia associated with use of tirzepatide.
SURPASS-3 and SURPASS-4 compared tirzepatide with insulin degludec and insulin glargine, respectively, in people with T2D who were taking oral hypoglycaemics (Ludvic et al, 2021; Del Prato et al, 2021). In both studies, tirzepatide achieved greater reduction in HbA1c than the basal insulin, and facilitated significant weight loss, in contrast to weight gain with the basal insulin. The study population in SURPASS-4 was at high cardiovascular risk and, reassuringly, tirzepatide was not associated with increased cardiovascular events and did induce significant benefit to lipid profile and blood pressure.
In SURPASS-5, tirzepatide was added to a basal insulin with or without metformin. HbA1c reduction and weight loss versus placebo were comparable to the earlier SURPASS studies (Dahl et al, 2022).

Tirzepatide – trials for obesity
The SURMOUNT randomised controlled trials looked at the effectiveness of tirzepatide in treating obesity, with weight reduction as the primary outcome. The findings from SURMOUNT-1 (Jastreboff et al, 2022) and SURMOUNT-2 (Garvey et al, 2023) are summarised in Table 5.
SURMOUNT-3 and SURMOUNT-4 are DBRCTs comparing weight change in tirzepatide (10 or 15 mg weekly) versus placebo in obese/overweight subjects without T2D. Formal reporting on these trials are awaited, but early reports have been issued (Park, 2023). In SURMOUNT-3, 12 weeks of intensive lifestyle intervention led to a mean 6.9% weight loss, following which there was randomisation to tirzepatide or placebo. An additional 21.1% mean weight loss with tirzepatide was achieved over 72 weeks (versus 3.3% weight regain with placebo), yielding a total mean weight loss of 26.6% with tirzepatide from study entry over 84 weeks.
With SURMOUNT-4, a 21.1% mean weight loss was achieved with tirzepatide over an initial 36-week open-label period, followed by an additional 6.7% weight loss over a further 52 weeks in the group randomised to receive continued tirzepatide (compared to 14.8% weight regain with placebo), giving a total mean body-weight loss of 26% in those who remained on tirzepatide therapy at 88 weeks.
In the SURMOUNT trials, tirzepatide use led to improvements in systolic and diastolic BP, lipid profile, waist circumference, liver enzymes and fasting insulin levels (thus indicating an improvement in insulin sensitivity).
As with the SURPASS trials (and trials with GLP-1 RAs), gastrointestinal symptoms were the predominant side effect, classified as mild to moderate in severity, mostly linked with dose escalation and decreasing over time. There were no increases in diabetic retinopathy events or episodes of pancreatitis versus placebo, and no cases of medullary thyroid cancer were reported. Cholecystitis was reported more frequently in the tirzepatide group than the placebo group in SURMOUNT-1, but incidence was low.
Tirzepatide – future trials
A direct comparison of the efficacy of tirzepatide versus semaglutide (as Wegovy 2.4 mg once weekly) in generating weight loss in people with obesity or overweight without T2D is planned in SURMOUNT-5
CVOTs of tirzepatide in subjects with and without T2D are ongoing. There are plans to investigate the effect of tirzepatide in sleep apnoea, heart failure and non-alcoholic steatohepatitis (NASH).
Tirzepatide – licensing and NICE guidance
Tirzepatide is now licensed for use in T2D when diet and exercise provide insufficient for glycaemic control (SmPC, 2023a). NICE (2023b) recommends considering tirzepatide for insufficiently controlled T2D in adults alongside lifestyle measures when:
- Triple oral therapy is ineffective, poorly tolerated or contraindicated, and
- They have a BMI of ≥35 kg/m2, and associated medical/psychological problems, or
- BMI <35 kg/m2 and insulin therapy would have significant occupational implications or weight loss would benefit other significant obesity-related complications.
BMI thresholds should be lowered by 2.5 kg/m2 for ethnic minorities.
It is noteworthy that the ADA/EASD consensus report places greater emphasis on use of GLP-1 RAs for T2D (Davies et al, 2022).
Tirzepatide has also recently gained a licence for weight management in people with BMI >30 kg/m2, and for the range 27–30 kg/m2 if there is one weight-related comorbidity (hypertension, dyslipidaemia, cardiovascular disease, obstructive sleep apnoea, prediabetes or T2D; SmPC, 2023b). NICE guidance on this issue is awaited.
Glucagon receptor agonism to facilitate weight loss
Glucagon is a peptide secreted from alpha cells within the pancreas. It functions as a counter-regulatory hormone to insulin, increasing glucose levels. Thus, the idea of utilising glucagon RAs for metabolic disease would seem counterintuitive, at least in people with diabetes. However, glucagon does inhibit food intake, inducing weight loss through activity at the glucagon and GLP-1 receptors. Glucagon is also thought to increase energy expenditure, possibly via increased brown adipose tissue activity (Baggio and Drucker, 2021).
Preclinical and clinical studies, in fact, demonstrate that glucagon receptor agonism in combination with GLP-1 receptor agonism reduces glucose levels and enables weight loss. The question, as with combining GLP-1 and GIP receptor activity, is whether adding glucagon receptor activity to GLP-1 receptor activity exceeds the glycaemic lowering and weight loss achieved with a GLP-1 RA alone (Ambury et al, 2018; Tiller et al, 2019).
Adding in glucagon receptor agonist activity
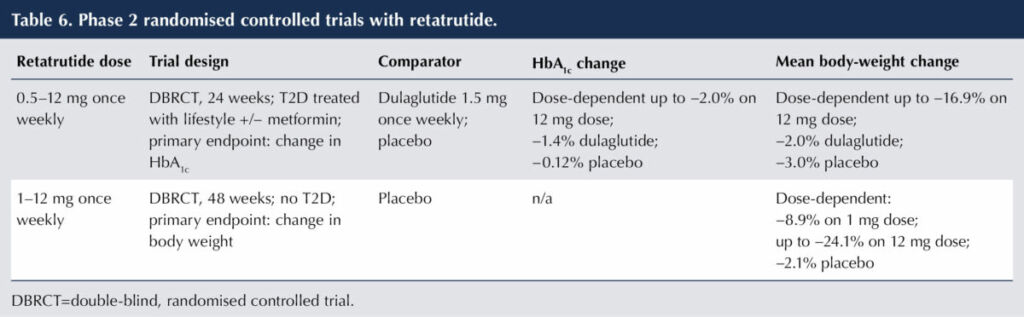
A clinically advanced molecule possessing glucagon receptor activity is the triple co-agonist retatrutide – a single peptide molecule combining GLP-1, GIP and glucagon RA activity, delivered subcutaneously once weekly. The results of phase 2 (dose-ranging) trials with primary endpoints of HbA1c change (Rosenstock et al, 2023) and body-weight change (Jastreboff et al, 2023) are shown in Table 6.
Mild-to-moderate gastrointestinal side effects were reported and were less prominent with titration up from a lower dose. Dose-dependent increases in heart rate were noted, peaking at 24 weeks. It has been pointed out that better active comparators than dulaglutide would be more powerful GLP-1 or GLP-1/GIP RAs (Bain and Min, 2023).
Survodutide is a dual co-agonist combining properties of GLP-1 and glucagon receptor agonism in a single peptide molecule that has also been evaluated in a phase 2 study for weight loss. In a randomised controlled trial with overweight/obese people, once-weekly survodutide induced dose-dependent mean body-weight changes up to −14.9% for those assigned to the maximum dose of 4.8 mg, compared to −2.8% with placebo. In this arm of the study, those participants who actually achieved and continued on the 4.8 mg dose weight reduction reached 18.7% at week 46 (Le Roux et al, 2023). Side effects with survodutide were in line with those expected for GLP-1 RAs and were the principal cause of discontinuation – 24.6% in the survodutide groups versus 3.9% with placebo. As most discontinuations occurred during the rapid dose-escalation period of treatment, slower up-titration of dose may counter these.
Conclusions
We now have available once-weekly injectable GLP-1 RAs with powerful efficacy both in control of hyperglycaemia and weight reduction. Additional benefits include improved cardiovascular outcomes in those with or at high risk of cardiovascular disease, and renoprotection. Gastrointestinal side effects are common and can lead to treatment discontinuation, but they can be mitigated by gradual dose up-titration.
The licensing of oral semaglutide was an important therapeutic advance offering an alternative to injectable GLP-1 RAs. However, the restrictions around taking oral semaglutide are burdensome. Thus, the search for non-peptide oral GLP-1 RAs to overcome absorption difficulty, and the progress of orforglipron without these administration restrictions, are very welcome.
Apart from their utility in the management of T2D, injectable liraglutide and semaglutide have gained licences for the management of overweight/obesity in people without T2D at higher doses than used for treatment of T2D.
The SURPASS and SURMOUNT trials demonstrate the effectiveness of tirzepatide in improving glycaemic control and promoting weight loss in obese individuals with and without T2D, approaching levels seen from bariatric surgery. They support the concept of additive benefit from using GIP and GLP-1 RAs in combination. The safety profile of tirzepatide is comparable with that of GLP-1 RAs. It is now licensed for use in T2D and for weight management.
A further avenue of innovation is that of unimolecular co-agonists incorporating glucagon receptor activity.
Amid the excitement of these new developments it is important to remember that attention to lifestyle factors remains the most fundamental means of tackling both T2D and obesity. Intensive lifestyle intervention has been shown to generate weight loss leading to remission of T2D (Lean, 2018; Lean, 2019). A tactful and encouraging approach to addressing diet and exercise, that is achievable and sustainable for the individual, is central to management strategy.
Other medications that can facilitate weight loss in T2D, notably the SGLT2 inhibitors, may prove more acceptable and better tolerated than GLP-1 RAs. Metabolic surgery can produce impressive reductions in body weight and remission of diabetes when deployed in carefully selected individuals.
An optimal strategy for managing people who are overweight/obese with or without T2D should consider a combination of interventions – lifestyle, medication and metabolic surgery.






Diabetes UK releases its 5-year strategy to tackle diabetes inequities.
16 Jul 2025