Managing blood glucose levels is the cornerstone of diabetes care, and it presents a long-term challenge for people with diabetes and healthcare professionals alike. There is a wealth of studies confirming the relationship between glycaemic control and adverse outcomes for people with diabetes.
Adoption of continuous glucose monitoring (CGM), including flash glucose monitoring, is increasing rapidly, both globally and in the UK, albeit UK growth is not uniform or linear. Within the diabetes professional community there is a definite shortage of skills related to diabetes technology, with varied uptake of both CGM and insulin pump therapy across the UK. CGM and the diabetes technology surrounding it have become increasingly sophisticated in recent years, and it is easy to see how healthcare professionals could have been left behind if they were not early adopters of the initial systems, creating a culture of those with tech knowledge and those without.
A standardised approach in this emerging field is much needed, so that we can focus on how best to interpret blood glucose data and realise the benefits in the real world.
Towards a standardised approach
It is no surprise that, with all the new technology and a limited evidence base, even those in the know – the experts, so to speak – cannot always reach universal agreement on how best to use CGM in the real world. However, there are now consensus recommendations for use of key CGM metrics that have been presented in three separate peer-reviewed articles (Danne et al, 2017; Petrie et al, 2017; Battelino et al, 2019), although formal adoption by diabetes professional organisations and official guidance has been absent for some time.
This article looks to outline a clinical strategy for CGM use and interpretation of the resulting data, and to provide accessible, practical tips for both healthcare professionals and CGM users.
Metrics of glucose control
In order to predict and shape future strategies, it is illuminating to look at both past and current practice and metrics. HbA1c has long been the main focus and target metric employed by clinicians and in CGM studies, and people with diabetes are also conditioned by such behaviours, after years of using HbA1c to monitor how well their blood glucose is being managed. This recognises the well-known relationship between HbA1c and the development of long-term complications.
HbA1c reflects the average glucose level over the past 60–90 days, but it is not able to quantify the number of hypoglycaemic events or the extent of glucose excursions (Beck et al, 2019). Furthermore, other medical conditions, including haemoglobinopathies, iron deficiency, pregnancy and chronic kidney disease, impair the reliability and utility of the test. Despite this, the use of HbA1c in clinical practise is set to remain, as it is still the only prospectively evaluated marker for risk of long-term diabetes complications.
Time In Range
HbA1c targets are embedded in diabetes practice, but the benefits of CGM and the ability to show live glucose data and other parameters of glucose variability lend themselves to a new focus and clinical narrative.
Chief among these new parameters is Time In Range (TIR): the amount or proportion of time per day spent with blood glucose levels between, according to international consensus, 3.9–10.0 mmol/L (Battelino et al, 2019).
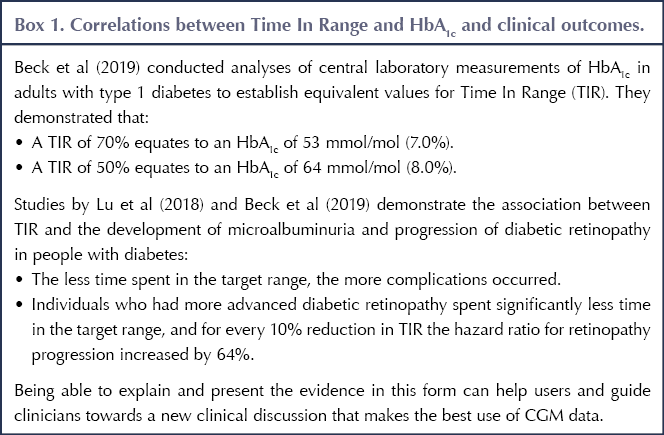
Recent studies suggest that TIR is closely associated with both HbA1c and clinical outcomes (see Box 1). Using these correlations, healthcare profesionals could say to CGM users that, for example, a TIR of 70% (i.e. 70% of time spent with blood glucose levels 3.9–10.0 mmol/L) equates to an HbA1c of 53 mmol/mol (7.0%); or that a 10% increase in TIR would reduce their HbA1c by 5 mmol/mol.
Core metrics for consultations
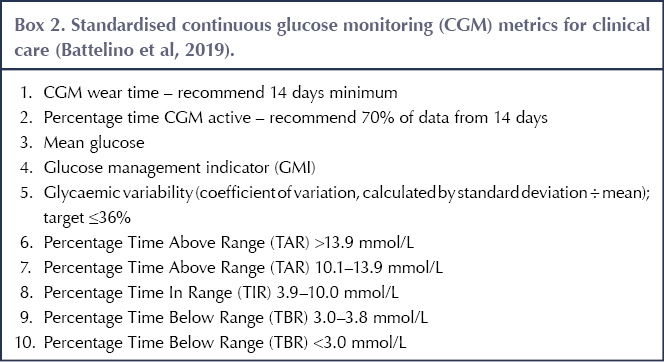
In October 2017, the Advanced Technologies and Treatments for Diabetes (ATTD) organisation set about producing core metrics for CGM data. They decided on 14 metrics but streamlined these to 10 for use in real-world clinical practice. These are shown in Box 2 (Battelino et al, 2019). By integrating these metrics into clinical consultations, a standardised approach is more achievable, as the metrics provide key indicators for users and healthcare professionals to work towards.
CGM allows identification of TIR, Time Below Range (TBR) and Time Above Range (TAR), but the new terminology alone represents a challenge. People with diabetes are used to talking in terms of “hypos” and “hypers”, or “running high”, as ways of describing TBR and TAR. Some of the challenges of understanding these new terms can be overcome by the graphical visualisation of the glucose data; nonetheless, when analysing the CGM data and discussing individual targets, it is important that we speak the same language.
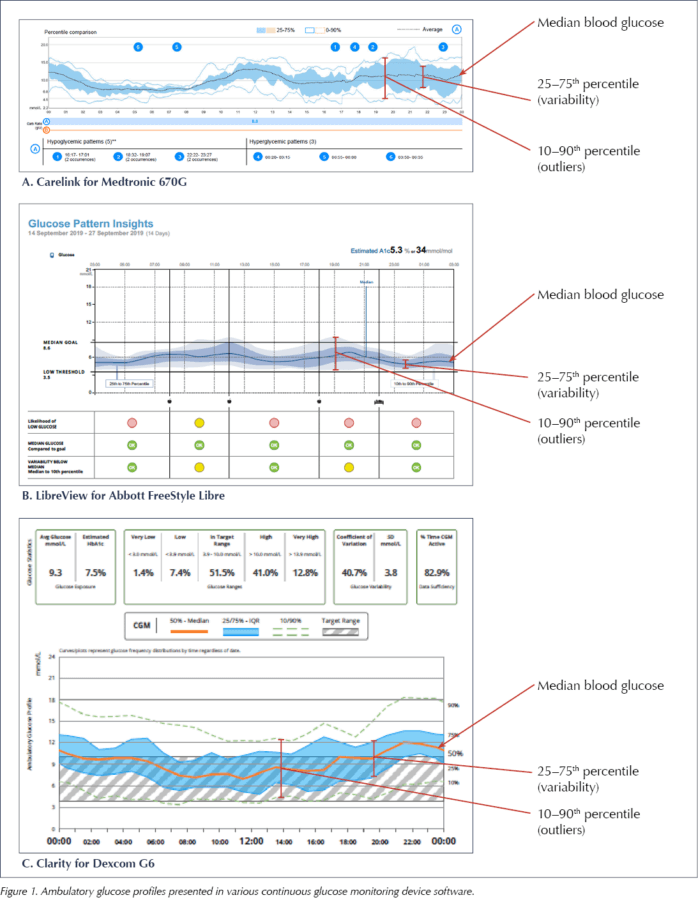
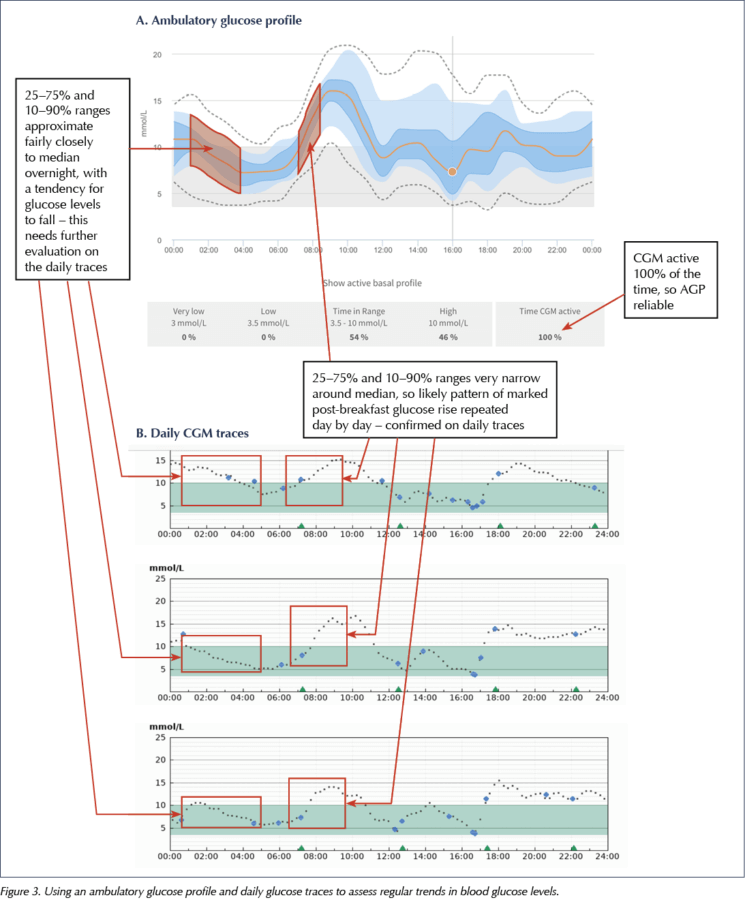
Most manufacturers have standardised Ambulatory Glucose Profile (AGP) software, which offers a reproducible display of glucose data (Figure 1). This allows rapid interpretation of daily glucose trends.
The AGPs in Figure 1 display multiple days of data over a single 24-hour plot, and clearly show the variation of glycaemic control in a colour-coded, eye-friendly way.
It is tempting to focus on the AGP as the graphical illustration of typical glycaemic patterns and, therefore, make adjustments to the treatment regimen purely on the basis of this information. However, at time points where there is a lot of variation around the median glucose, particularly after meals, it is important to remember that this is just an average of glucose levels at that time point and that the trend in the median line is an average of different daily trends.
The AGP is thus better used to allow visualisation of variability and TIR, giving an overview of blood glucose trends, and to direct the user or clinician to times of day where there is significant variation, in which case drilling down into the daily glucose traces may be informative to assist changes in the treatment regimen.
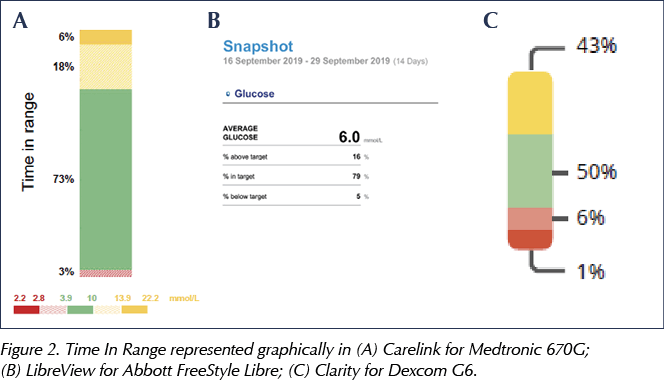
What the AGP does allow is easy identification of TIR, as well as glucose excursions, and this could sensibly be offered as the main clinical topic of discussion. Figure 2 shows how TIR is displayed in three different sensors.
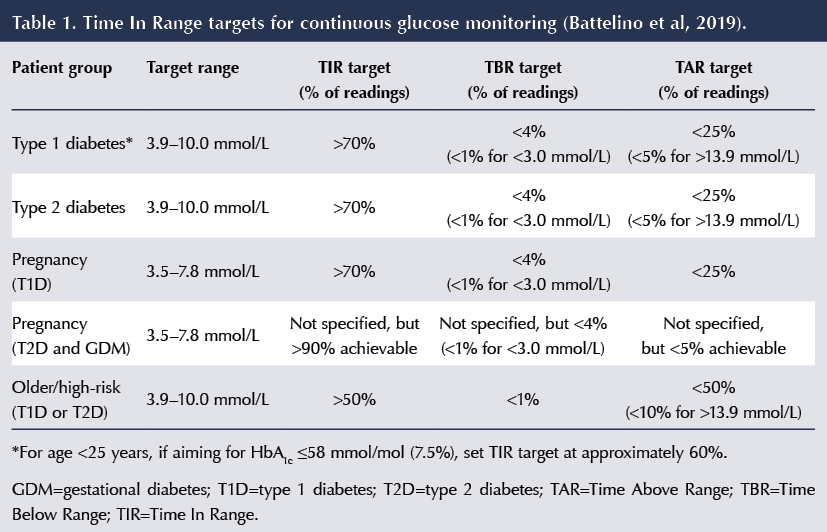
Targets for TIR, TBR and TAR
The targets for TIR, TBR and TAR recommended by Battelino et al (2019) are as follows (see Table 1 for a brief summary).
- In people with type 1 and type 2 diabetes, the TIR target is to achieve at least 70% of time at 3.9–10 mmol/L.
- In pregnant women with type 1 diabetes, TIR can be used as a marker to promote a safe but rapid improvement in blood glucose levels. The target for TIR remains at least 70%, but the range is narrowed to 3.5–7.8 mmol/L.
- There are more ambitious targets recommended for pregnant women with type 2 and gestational diabetes, in whom the risk of hypoglycaemia is considerably lower and 90% TIR is achievable, although a target TIR percentage was not specified in the consensus document.
- Less ambitious targets – 50% TIR – are recommended in older and higher-risk people with type 1 and type 2 diabetes.
Analysing CGM data: A structured approach
Whether on multiple daily injections or insulin pump therapy, use of CGM provides far more reliable and dynamic information, both retrospectively and prospectively, than has been available before. By reviewing the trends of the AGP, clinicians and users could conceivably adjust basal rates and bolus doses, as well as diet and exercise, in order to maintain glycaemic control.
We have generally advocated that the approach to analysis should be based on the following sequence:
- Individual daily profiles.
- Breaking the CGM traces into overnight, fasting/pre‐meal and post‐meal phases.
- Finally, looking at the impact of other factors such as exercise, alcohol and work patterns.
Given the recommendations regarding glucose metrics from CGM data and how these are presented alongside the AGP, this approach can be modified so that users can be educated to make changes to both their insulin regimen and lifestyle to optimise glucose control. A suggested approach could therefore look like this:
User history
Quality and quantity of data available. Look at number of days worn, aiming for a minimum of 14 days.
- Percentage of time CGM is active, aiming for a minimum of 70% of the time.
- What insulin; multiple daily injections or insulin pump; basal and bolus doses.
- Physical activity.
- Working or non-working days.
- Illness – any additional therapy (e.g. steroid treatment).
Ambulatory glucose profile
- Focus on TIR – glucose range 3.9–10.0 mmol/L.
– Discuss with users that 60% TIR represents reasonable good control, equivalent to an HbA1c of 58 mmol/mol (7.5%).
– A TIR of 70% indicates even tighter control, equivalent to an HbA1c of approximately 53 mmol/mol (7.0%). - Identify Time Below Range and assess the severity (i.e. how low do they go?).
– Discuss low readings and the potential causes.
– Advise users that the target is to spend less than 4% of the time – or 1 hour per day – with glucose levels in the range 3.0–3.8 mmol/L.
– Readings below 3.0 mmol/L have the potential to cause greater, clinically significant harm, so the target would be to spend less than 1% of the time (15 minutes per day) in this range. - Time Above Range:
– Aim to spend less than 25% (6 hours per day) with glucose over 10 mmol/L.
– Aim for less than 5% (1 hour and 12 minutes per day) with readings over 13.9 mmol/L.
Review AGP for each part of the day
- Overnight (See Figure 3 for examples).
- Morning meal (See Figure 3 for examples).
- Midday meal.
- Evening meal.
- Look at food intake – is the user counting carbohydrates?
- Physical activity – does it cause hypoglycaemia or glucose excursions?
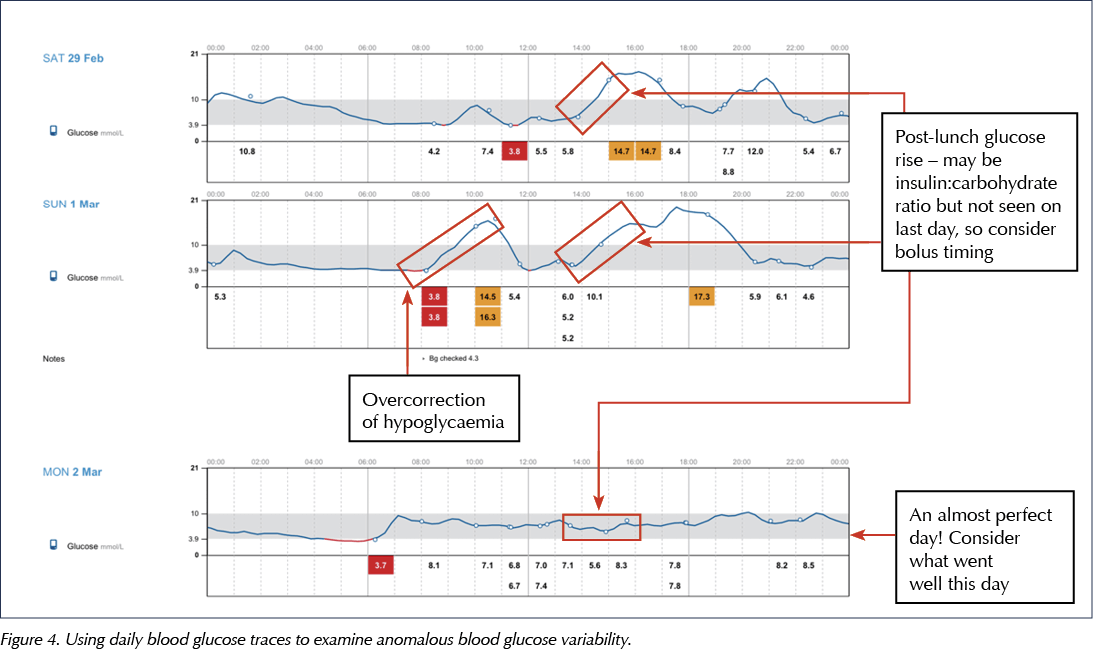
Assess for anomalous days
- Is there high glucose variability?
- May need to look at day-to-day analysis to establish any underlying issues (see Figure 4 for examples).
Recommendations to users
- Summarise the key AGP points.
- Reinforce one key message from the CGM data:
– Prioritise minimising hypoglycaemia.
– Then focus on Time Above Range.
– Then consider variability and identify causes (e.g. inaccurate carbohydrate counting, late meal bolus, exercise, stress). - Avoid initiating change without clear patterns – may need longer to collect data.
Summary
The optimal use of CGM in both academic and clinical practice is emerging and complex, and the availability and uptake nationally and internationally is highly variable. Despite considerable efforts to develop an international consensus as to how best to use CGM data, clear and concise guidelines and targets have yet to make an impact at the coal face, and a common approach is lacking in routine clinical practice.
By simplifying and streamlining the clinically relevant data, clinicians and users can use the more dynamic data that CGM makes available to guide management, helping the user to achieve more optimal glucose control, improving immediate quality of life and, in the longer term, reducing the risk of complications.



Developments that will impact your practice.
22 Dec 2025