Continuous glucose monitoring (CGM) has transformed diabetes management, offering a dynamic alternative to traditional self-monitoring of blood glucose (SMBG). While SMBG provides static snapshots, CGM delivers a continuous stream of data, enabling pattern recognition, real-time response to glycaemic trends and informed insulin dosing.
As of 2025, CGM is considered standard care for all individuals with type 1 diabetes using insulin therapy, as well as for specific groups of people with type 2 diabetes in the UK. This has been made possible through guidance and approval from NICE. This article aims to demystify CGM technology, explain its regulatory context and equip diabetes nurses with the knowledge needed to evaluate CGM systems in clinical practice.
National guidelines: The clinical imperative
Recent updates from NICE emphasise the pivotal role of CGM in insulin management:
- NG17: All adults living with type 1 diabetes should be offered CGM (NICE, 2022a).
- NG18: All children and young people with type 1 diabetes should be offered CGM (NICE, 2023a).
- NG28: For adults living with type 2 diabetes who are on multiple daily insulin injections, intermittently scanned CGM (isCGM) should be offered to individuals experiencing recurrent or severe hypoglycaemia; those with impaired hypoglycaemia awareness; and those with disabilities or cognitive impairments that prevent them from reliably using finger-prick blood glucose monitoring but who could benefit from scanning a sensor themselves or with support (NICE, 2022b). It should also be made available to individuals advised to test their glucose levels eight or more times daily.
Furthermore, isCGM is recommended for people with insulin-treated type 2 diabetes who require assistance from a healthcare professional or care worker to monitor glucose levels, supporting greater independence and reducing the burden on care services. In settings where real-time CGM (rtCGM) is available at the same or lower cost, it may be considered as an alternative for this group. - TA943: Hybrid closed-loop (HCL) systems are recommended for people living with type 1 diabetes, with national funding ring-fenced for five years to support access. Eligibility criteria include all children, and adults with an HbA1c of 58 mmol/mol or above, frequent hypoglycaemia, or those who are pregnant or planning to become pregnant (NICE, 2023b). CGM is a core component of HCL therapy, where real-time glucose levels and their rate of change drive automated insulin delivery adjustments.
These recommendations reflect a strong evidence base. CGM improves glycaemic outcomes, enhances safety and supports quality of life for those using insulin therapy (Maiorino et al, 2020). Furthermore, HCL systems offer additional glycaemic and quality-of-life benefits (Beck et al, 2023; Zeng et al, 2023).
Adjunctive vs. non-adjunctive use: Why it matters
Not all CGM systems are created equal. Devices fall into two main categories:
- Adjunctive CGM: Requires confirmation with finger-prick testing before insulin dosing.
- Non-adjunctive CGM: Approved for insulin dosing decisions without additional SMBG.
Understanding whether a CGM device is approved for insulin decision-making is essential. Nurses must remain vigilant when supporting people using CGM for this purpose. Currently, those using CGM for insulin dosing are all people who meet the NICE eligibility criteria (NICE, 2022a; 2022b; 2023a; 2023b).
CE and UKCA marking: Misunderstood signals
Devices sold in the UK must carry CE or UKCA marking, which confirms they meet general medical device minimum safety requirements. However, these markings do not indicate clinical accuracy or suitability for insulin dosing. In contrast, in the US, the evaluation and regulatory approval of CGM systems is overseen by the Center for Devices and Radiological Health (CDRH), a division of the Food and Drug Administration (FDA). The CDRH has established a higher standard through its “integrated CGM” (iCGM) designation and Class III (highest risk category) pre-market approval. Specifically, iCGM approval requires manufacturers to meet strict criteria for accuracy, reliability and interoperability (the ability to integrate safely with HCL and other devices; FDA, 2022).
A review of CGM regulations across Europe and the US highlights these key differences. While CE or UKCA marking is necessary for market access, it should not be interpreted as a guarantee of clinical quality. By comparison, FDA iCGM approval and Class III pre-market approvals reflect a level of clinical robustness and safety specifically suited for insulin decision-making (Pemberton et al, 2022).
This distinction is important for UK clinical teams to understand. With CGM options evolving rapidly, nurses play a critical role in supporting people with diabetes to use these devices appropriately and safely, especially when the devices are used to guide insulin dosing. In such cases, only non-adjunctive CGM systems with robust accuracy and safety data should be prescribed.
Study design: The foundation of trust
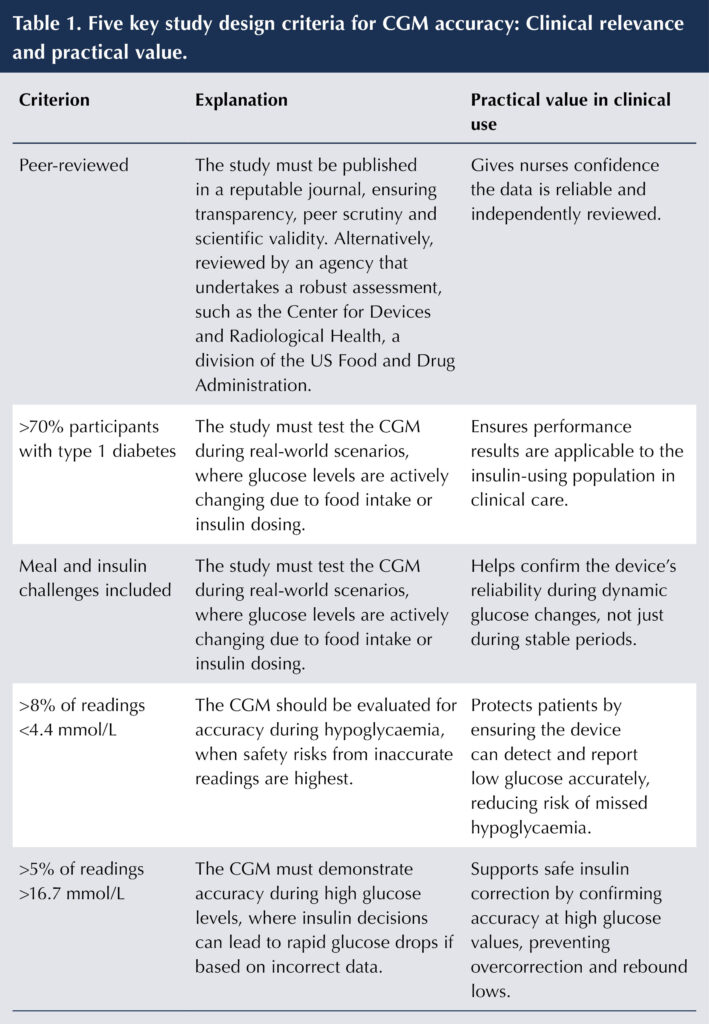
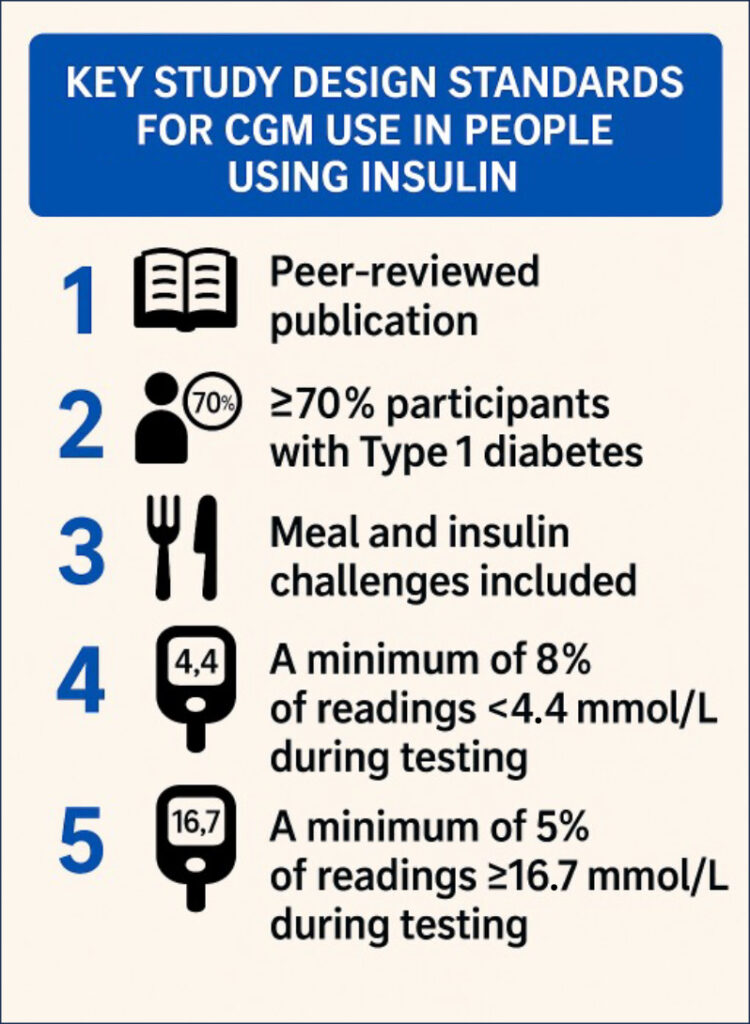
The reliability of a CGM for insulin dosing decisions depends on how it was tested. At a minimum, robust study design should meet five internationally accepted criteria (Table 1), first outlined in 2020 by the Clinical and Laboratory Standards Institute (CLSI, 2020). These criteria include that the data is peer-reviewed, the inclusion of more than 70% of participants with type 1 diabetes, the use of meal and insulin challenges to test device performance under real-world conditions, and evaluation across a broad glucose range, including both hypoglycaemic and hyperglycaemic episodes. These criteria have since been endorsed by both the International Federation for Clinical Chemistry (IFCC) Working Group for CGM (Freckmann et al, 2023) and a panel of clinical experts from across Europe (Mathieu et al, 2025). The checklist in Figure 1 serves to support study design assessment.
A simple analogy helps to clarify the importance of testing a CGM device across the measurement range, typically 2.2–22.2 mmol/L: Testing a CGM device only during stable glucose levels is like test-driving a car only on a straight, empty road. We must also evaluate how it handles curves, hills and traffic, the real-world challenges of glycaemic variability.
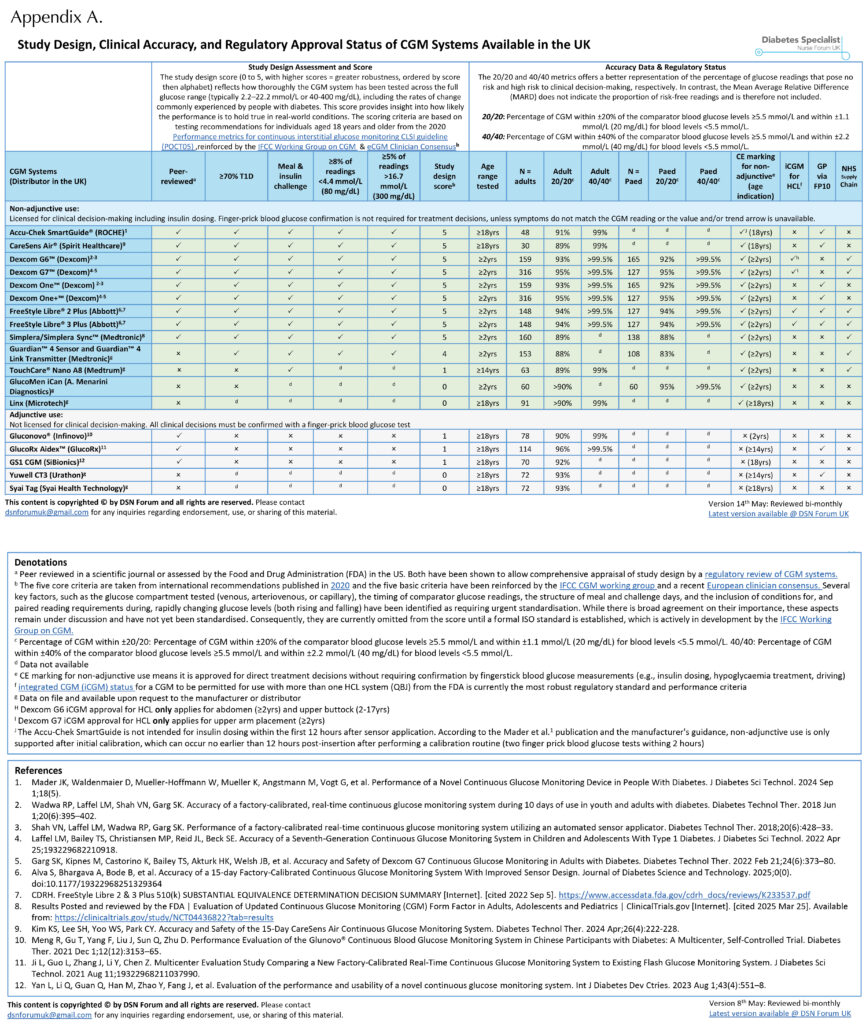
Appendix A shows the CGM devices currently available in the UK that are licensed for insulin dosing, along with their study design score (out of 5). This score reflects the clinical relevance of the performance data for insulin dosing decisions supporting each device.
Lower scores may reflect limited testing in people who use insulin, making it harder to evaluate how well the CGM performs in this group who often experience rapid and wide fluctuations in glucose levels throughout the day.
In contrast, higher scores suggest that the device has been more thoroughly evaluated in individuals who use insulin, offering greater confidence in its performance for this group who often experience marked glucose variability. This includes situations such as rapid rises in glucose after meals without insulin coverage, or sharp declines following large correction doses, scenarios where accurate CGM readings are especially critical for safe insulin management.
Accuracy metrics: Looking beyond MARD
Once study quality for insulin users is confirmed, the next step is to interpret accuracy metrics. The most cited is mean absolute relative difference (MARD), which reflects the average variance between CGM readings and reference glucose values. A MARD under 10% is generally considered acceptable for insulin dosing (Kovatchev et al, 2009).
However, MARD is an average value and may mask clinically significant inaccuracies at the extremes of hypoglycaemia or hyperglycaemia (Heinemann et al, 2020). Nurses should be cautious when interpreting a low MARD, especially if it is presented without details about the study design, such as whether the study included people using insulin or if the testing conditions reflected real-world glucose fluctuations (see Table 1 and Figure 1).
A more clinically relevant accuracy measure: The 20/20 and 40/40 metrics
The 20/20 accuracy metric offers a clinically meaningful assessment of CGM performance by specifying how close the true blood glucose (laboratory or accurate SMBG reading) value must be to the CGM reading to consider it accurate. The thresholds depend on the CGM value:
- If the CGM reading is <5.5 mmol/L, the true blood glucose must fall within ±1.1 mmol/L (±20 mg/dL) of the CGM value.
- For example, a CGM value of 4.5 mmol/L would be considered accurate if the reference glucose lies between 3.4 and 5.6 mmol/L.
- If the CGM reading is ≥5.5 mmol/L, the true value must fall within ±20% of the CGM value.
- For example, if the CGM shows 10.0 mmol/L, the true glucose must be between 8.0 and 12.0 mmol/L to qualify as accurate.
A higher proportion of readings meeting the 20/20 criteria indicates stronger CGM reliability for safe insulin dosing.
The 40/40 accuracy metric provides a broader assessment of CGM performance. It is particularly useful for identifying the percentage of readings that fall outside this threshold and could lead to inappropriate insulin dosing decisions.
- If the CGM reading is <5.5 mmol/L, the true blood glucose must fall within ±2.2 mmol/L (±40 mg/dL) of the CGM value.
- For example, if the CGM reads 4.5 mmol/L, the paired true glucose must be between 2.3 and 6.7 mmol/L to meet the 40/40 accuracy threshold.
- If the CGM reading is ≥5.5 mmol/L, the true value must be within ±40% of the CGM reading.
- For example, if the CGM shows 10.0 mmol/L, the matched glucose must fall between 6.0 and 14.0 mmol/L to meet the 40/40 accuracy threshold.
The 40/40 metric provides insight into the proportion of readings that fall outside this wider threshold, highlighting how many could result in problematic insulin dosing, such as mistreatment of hypoglycaemia or unnecessary correction.
Appendix A shows the percentage of CGM readings that meet the 20/20 and 40/40 accuracy agreement rates across all currently available CGM devices, along with their regulatory status (adjunctive or non-adjunctive).
It is important to note that direct comparisons between CGM systems are limited due to differences in study design. Rather than serving as a direct head-to-head comparison, Appendix A should be interpreted as a guide to identify which CGM systems meet clinically meaningful design standards. The five criteria used to generate the study design score (Table 1) provide a useful starting point, but many additional factors influence the reliability of performance data. These include whether the CGM was validated against venous or capillary blood glucose values, whether meal and insulin challenges were performed on the same day, and the number of sensor lots tested, among others (Freckmann et al, 2023; Pleus et al, 2024).
In addition, there are multiple ways to assess CGM accuracy. These include the iCGM 15/15 and 40/40 metrics, four types of error grid analyses, and assessments of trend arrows and alert performance (Pemberton et al, 2022). The IFCC Working Group is currently developing a standardised approach for evaluating CGM accuracy specifically for insulin dosing decisions (Pleus et al, 2024). This effort aims to align with ISO standards to produce internationally recognised benchmarks similar to those already used for SMBG devices (Jendrike et al, 2019).
Appraising a CGM system: A practical framework
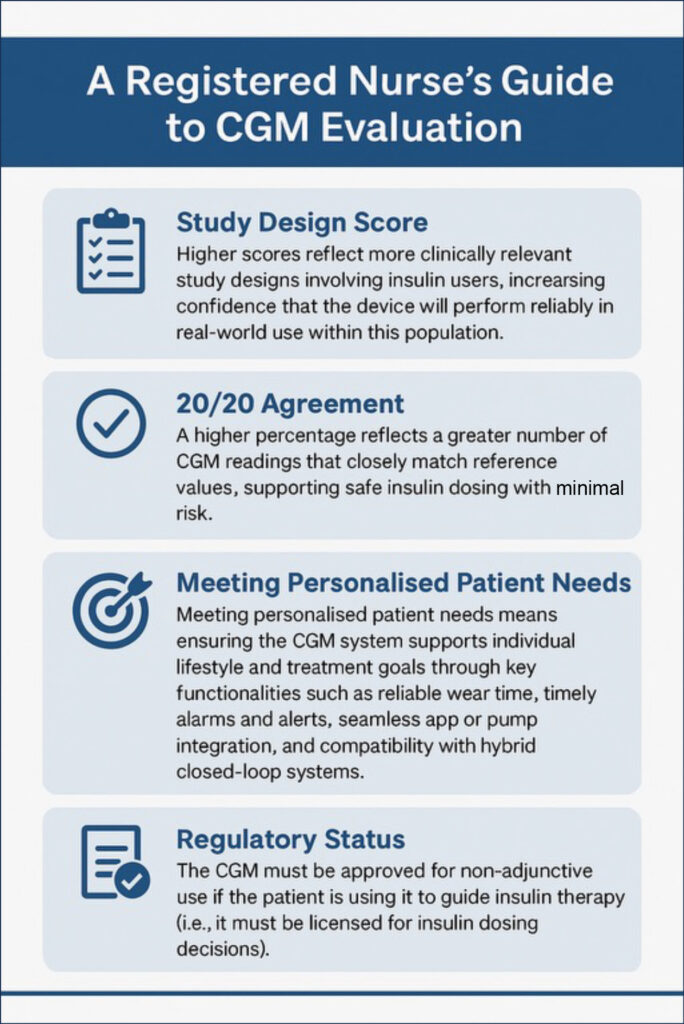
When assessing a CGM system for someone using insulin, it is important to consider the following key factors (see Figure 2):
- Study design score: Higher scores reflect more clinically relevant study designs involving insulin users, increasing confidence that the device will perform reliably in real-world use within this population.
- 20/20 agreement: A higher percentage reflects a greater number of CGM readings that closely match reference values, supporting safe insulin dosing with minimal risk.
- 40/40 agreement: A higher percentage reflects better CGM accuracy, indicating a low number that fall outside the threshold and may cause insulin dosing errors.
- Meeting personalised patient needs: Ensuring the CGM system supports individual lifestyle and treatment goals through key functionalities, such as reliable wear time, timely alarms and alerts, seamless app or pump integration, and compatibility with hybrid closed-loop systems.
- Regulatory status: The CGM device must be approved for non-adjunctive use if the patient is using it to guide insulin therapy (i.e. it must be licensed for insulin dosing decisions).
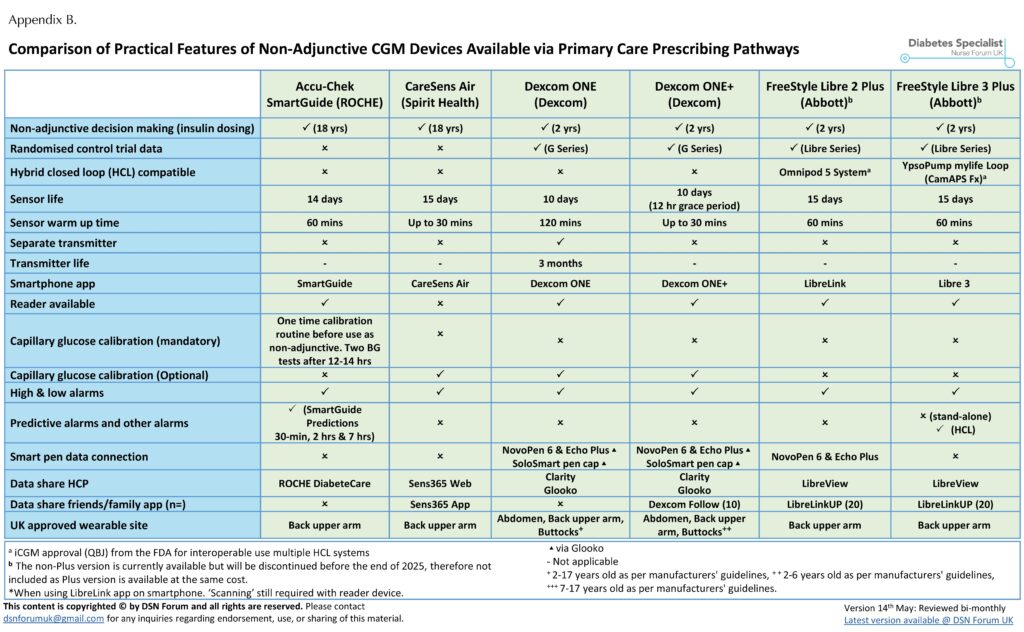
Devices that are not approved for non-adjunctive use must never be used alone to guide insulin dosing decisions. This has important implications in primary care, where some lower-cost CGM systems without non-adjunctive approval are still available through prescribing systems (GP via FP10; Appendix A).
Prescriber awareness of these regulatory distinctions may vary, highlighting the need for clear guidance to ensure patients are using clinically appropriate devices for insulin management. Additionally, the inclusion of these devices in primary care prescribing systems may warrant further consideration. NICE only recommends CGM for people with type 1 diabetes and for those with type 2 diabetes (specific criteria) who are using insulin (NICE, 2022a; 2022b; 2023a; 2023b).
Practical features: Matching tech to patient needs
After accuracy and regulation, practical usability comes into focus. Registered nurses should guide patients through options such as:
- Sensor wear time (7, 14 or 15+ days) and wear sites.
- Calibration requirements.
- Alarm and alert options with available customisation.
- Smartphone and smartpen integration.
- Compatibility with HCL systems.
- Data-sharing capabilities.
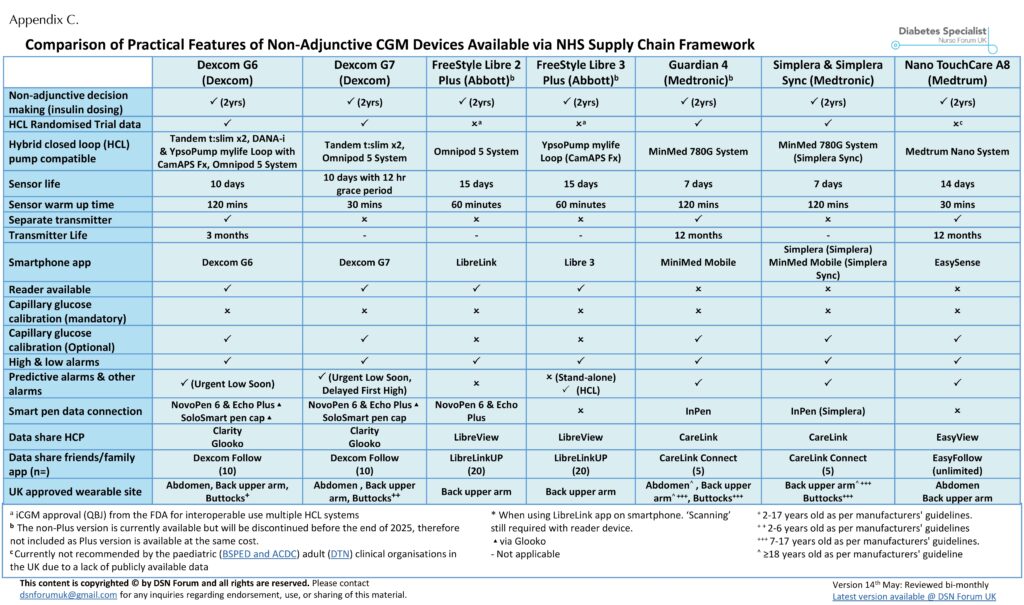
Choosing the right CGM device depends on the patient’s lifestyle, preferences and clinical goals. The DSN Forum UK website (www.diabetesspecialistnurseforumuk.co.uk) provides a regularly updated comparison of CGM device study designs (Appendix A) and their features, including those available for insulin dosing on prescription in primary care (Appendix B) and through the NHS Supply Chain (Appendix C). This resource has been officially endorsed by Breakthrough T1D and Diabetes UK.
This article does not aim to cover all available device features in detail. For the latest updates on technology and prescribing options, readers are encouraged to visit the DSN Forum website, where the DSN Forum UK team provides ongoing guidance as the landscape continues to evolve.
Hybrid closed-loop systems: The importance of CGM interoperability and accuracy
HCL systems represent a significant advancement in diabetes technology, using continuous data from CGM devices to automatically adjust insulin delivery via an algorithm and pump. With HCL therapy now recommended by NICE (2023b) for eligible individuals with type 1 diabetes, the choice of CGM within the system becomes a clinically critical decision.
iCGM: A regulatory benchmark for interoperability and accuracy
The FDA’s iCGM designation provides a benchmark for CGM systems that meet high standards for accuracy, reliability and, crucially, interoperability with HCL systems. Sensors certified as iCGM (code QBJ) must meet predefined accuracy thresholds across the glucose range, maintain consistent performance over multiple sensor lots and prove their compatibility with automated insulin delivery (AID) systems (FDA, 2022).
Devices with iCGM certification are, therefore, uniquely suited to HCL use, with demonstrated accuracy under real-world conditions and a clear regulatory pathway ensuring they can safely integrate with third-party algorithms and pumps. Appendix A shows that the Dexcom G6 and G7, as well as FreeStyle Libre 2 Plus and Libre 3 Plus, all have iCGM (code QBJ) approval. These sensors are those used within widely adopted HCL systems, including CamAPS FX®, Tandem t:slim with Control-IQ® and Omnipod® 5 System. All of these HCL systems have multiple trials and real-world data, confirming their safety and efficacy (Phillip et al, 2023).
Medtronic: An alternative regulatory pathway with strong outcome data
Medtronic CGM systems, such as those used in the MiniMed™ 780G, are not iCGM-certified. Instead, these sensors are approved as Class III devices by the FDA. They do not meet the technical requirements for iCGM designation (including external interoperability). However, these systems are approved as part of an HCL system as they are supported by robust trial and real-world data (Phillip et al, 2023). Therefore, despite not having iCGM status, Medtronic’s system remains a trusted and evidence-backed choice in HCL therapy.
TouchCare® Nano A8: Used in HCL but lacking peer-reviewed data
By contrast, the TouchCare® Nano A8 sensor (used in the Medtrum Nano HCL system) has a low study design score (Appendix A), and the HCL lacks publicly available trial data or peer-reviewed real-world data at the time of approval and to date. Although it has received CE marking and is available under NICE TA943 (NICE, 2023b), the absence of published clinical evidence presents challenges for clinicians seeking to assess its appropriateness and safety.
This does not suggest that the Medtrum HCL system is unsafe, but rather highlights the lack of transparent, publicly available performance data. As a result, professional bodies, including the Diabetes Technology Network UK (2024), and a joint statement from the British Society for Paediatric Endocrinology and Diabetes and the Association of Children’s Diabetes Clinicians (BSPED/ACDC, 2024), have recommended caution and called for additional data.
CGM for use in people without diabetes: Proceed with caution
CGM use is expanding beyond diabetes into the wellness market, with consumers using sensors to monitor glucose trends for lifestyle and health optimisation. While there is potential benefit, first through an initial learning phase to support behaviour change, followed by a long-term accountability phase to help sustain those changes, it remains unclear whether these devices offer the level of precision needed to reliably track glucose within tighter, normal glycaemic ranges (Oganesova et al, 2024).
In individuals without diabetes, and those with non-diabetic hyperglycaemia (NDH, previously known as pre-diabetes), glucose levels generally range between 3.3 and 10.0 mmol/L with relatively low variability. In this context, even small inaccuracies in CGM readings can result in false hypoglycaemia alerts or falsely elevated readings above the 11.1 mmol/L diagnostic threshold for diabetes. These misleading data points may cause unnecessary anxiety and lead to inappropriate dietary changes or restrictions (Oganesova et al, 2024).
The current 20/20 and 40/40 accuracy thresholds may not provide sufficient precision for individuals not living with diabetes. In this population, where glucose levels typically remain within a tighter range with little variation, a stricter standard may be more appropriate. For example, adopting a 10/10 threshold, requiring values to fall within ±0.6 mmol/L (±10 mg/dL) for readings <5.5 mmol/L, and within 10% for readings >5.5 mmol/L, could better reflect the level of accuracy needed. Additionally, using 20/20 as a threshold for identifying potentially problematic readings may offer more meaningful insights when CGM is used to support behavioural change in people not living with diabetes.
Although there is currently no consensus on the use of CGM in individuals without diabetes or those with NDH, its potential benefits are evident. However, the cost–benefit case for this use remains unclear. To move forward responsibly, it is essential to better understand the level of accuracy required in this population, and to test these expectations using robust study designs that reflect real-world conditions. While CGM may offer valuable insights, it may also carry unintended and poorly understood consequences. Meanwhile, a growing number of CE-marked CGM devices are now available for purchase online (see Appendix A), many of which are marketed specifically for wellness and NDH purposes.
The path forward: Regulation and quality labelling
Current CE and UKCA regulatory frameworks do not guarantee clinical accuracy of CGM systems (Pemberton et al, 2022). To address this gap, global efforts are underway, led by the IFCC Working Group on CGM (Pleus et al, 2024), to develop formal, CGM-specific accuracy standards. The goal is to establish an ISO-equivalent standard, similar to what already exists for SMBG (Jendrike et al, 2019).
A 2024 European consensus proposes a Europe-CGM (eCGM) designation for devices meeting rigorous real-world performance and transparency criteria (Mathieu et al, 2025). Such a designation would offer a short-term solution to help clinicians and people living with diabetes navigate an increasingly crowded CGM market with greater clarity and confidence, while we await the formal ISO standard.
Conclusion
Diabetes specialist nurses are at the forefront of integrating CGM into clinical care. To do this safely and effectively, we must move beyond surface-level indicators and develop a deep understanding of device testing, performance metrics and regulatory distinctions.
By using a structured approach to CGM evaluation – starting with study design, followed by performance metrics, regulatory approval status and patient-centred features – registered nurses working across primary care, secondary care, care homes and hospital settings can support personalised and evidence-based device choices.
The evolving comparison charts provided by the DSN Forum UK (https://www.diabetesspecialistnurseforumuk.co.uk), offer a valuable, regularly updated resource to help navigate the growing CGM market until a formal ISO standard is introduced, providing the community with the information necessary to evaluate efficacy and safety of CGM devices prescribed for insulin dosing decisions.
Contributions
AW: Design, background research, data collection, manuscript writing; BK: Data collection and manuscript review; TFS: Data collection and manuscript review; JP: Design, background research, manuscript writing, intellectual revision.
All authors were involved in the approval of the final version for publication.
Conflicts of interest
AW: Speaker payments for Roche, Abbott, Dexcom, mylife, Glooko, Air Liquide and Insulet.
BK: Speaker payments for Roche, Abbott, Dexcom, mylife, Glooko, Urathon, Air Liquide and Insulet.
TFS: Speaker payments for Roche, Abbott, Dexcom, mylife, Insulet, Glooko, Air Liquide and Medtronic.
JP: Advisory Board for Roche and Abbott, and speaker payments for Abott, Dexcom and Insulet.
Funding statement
This work did not receive any specific funds.
Acknowledgments
We would like to gratefully acknowledge Christina Lange-Ferreira and Dr Rebecca Thomas for proofreading the final draft.






Developments that will impact your practice.
29 Aug 2025