Vegetarian and vegan diets have been associated with several health benefits, including lower risk of certain types of cancer and cardiovascular disease, lower blood lipid levels and lower blood glucose levels, as well as better management of diabetes overall (Dinu et al, 2017). A number of epidemiological studies have shown that high consumers of fruits, vegetables and fibre have a reduced risk of type 2 diabetes (InterAct Consortium, 2015; Dinu et al, 2017). A 2009 analysis of the Adventist Health Study-2 revealed that the prevalence of type 2 diabetes amongst vegans was 2.9% as opposed to 7.6% among non-vegetarians (Tonstad et al, 2009). This was partly due to the significantly lower BMI in vegans; however, the association remained significant even after adjusting for BMI.
Insulin resistance and impaired insulin production are the driving force in the development of type 2 diabetes. Higher-fat diets have been shown to increase the accumulation of intramyocellular lipids, making people susceptible to insulin resistance (Sakurai et al, 2011). In addition to their lower fat content, plant-based diets offer protection against type 2 diabetes due to their high antioxidant, polyphenol, fibre and micronutrient composition. A systematic review identified 111 plants that have the ability to improve insulin sensitivity (Eddouks et al, 2014). Accumulation of fat in the cells of the pancreas can have detrimental effects as it can lead to impaired beta-cell function (Heiskanen et al, 2018). Exercise is a known contributor of improved beta-cell function; however, diet may also exert beneficial effects on pancreatic beta-cells by reducing pancreatic fat mass, lowering hyperglycaemia and reducing hyperlipidaemia (Larson-Meyer et al, 2006).
The production of insulin by beta-cells is also impaired by the presence and accumulation of advanced glycation end-products (AGEs) which are produced from precursor molecules such as methylglyoxal. Methylglyoxal has been shown to induce oxidative stress and cell death in pancreatic cells (Su et al, 2017). AGEs are formed endogenously in the body; however, the preparation of animal-derived products such as meat and cheese with the use of high heat results in the formation of AGEs in concentrations which are much higher than in plant-based foods (Uribarri et al, 2010). Furthermore, insulin resistance increases the secretory demand on beta-cells, which results in a decline in beta-cell function (Cerf, 2013). Therefore, dietary interventions that are low in animal fat and contain high amounts of fibre and antioxidants may lead to an improvement in the regulation of fat and glucose homeostasis, thereby preserving or improving beta-cell function.
Animal and in vitro studies have shown that factors such as caloric restriction (fasting) and plant chemicals such as flavonoids may lead to the preservation and regeneration of islet beta-cells, and thus improvement or restoration of insulin synthesis (Cheng et al, 2017; Ghorbani et al, 2019). However, the number of human studies involving plant-based dietary interventions are limited, and there have not been any systematic reviews analysing these studies.
Collectively, there is sufficient evidence to suggest that plant-based diets may directly impact the insulin-producing beta-cells of the pancreas. There are several systematic reviews and meta-analyses evaluating the efficacy of vegan or vegetarian diets in the treatment or prevention of type 2 diabetes; however, none of the reviews directly focus on the effect of plant-based diets on beta-cell function. Therefore, this study was conducted to evaluate these effects.
Methods
This systematic review was registered on PROSPERO (CRD42020168191) and has been conducted in line with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement.
A comprehensive and systematic literature search was conducted in an effort to identify relevant randomised controlled trials or interventional studies. A search strategy was devised using the PICO method for search term generation, which was used to search the Medline, EMBASE and Scopus databases. The key words used in combination as MeSH terms and text words were as follows: “Obesity” OR “obese” OR “Diabetes Mellitus” AND “plant based” OR “plant-based” OR “plant based” OR “plant food” OR “plant food” OR “vegan” OR “vegetarian” AND “beta cell function” AND “beta-cell function” AND “b-cell function” AND “insulin secretion” AND “pancreatic function” AND “β cells”. The systematic search was limited to human studies.
The references of included studies were also manually searched for potentially relevant articles.
Inclusion/exclusion criteria
Studies were included if they met the following inclusion criteria:
- Study designs had to be randomised controlled trials or non-randomised dietary intervention trials.
- Studies had to have a plant-based or vegan diet intervention group.
- Studies had to report C-peptide or insulin concentrations at baseline and post-intervention.
Studies were excluded if they were not published in a peer-reviewed journal. Studies that had enrolled participants with type 1 diabetes, were either ongoing or unpublished, did not include a plant-based intervention or were published in a non-English language were also excluded.
Data extraction
Two reviewers utilised a data extraction form adopted for this systematic review to extract all relevant data from the articles that met the inclusion criteria. The data extraction form was adopted from the SIGN (2015) data extraction form.
The systematic search of the literature was carried out by RKJ, who also screened relevant abstracts and titles, and completed data extraction. A second author, CN, was involved in data extraction. Any disagreement during data extraction was resolved by consensus.
The following data were extracted from relevant articles: author and publication date, country, participant characteristics relating to baseline health status (i.e. presence or absence of obesity or type 2 diabetes), sample size, intervention diet, control diet, measure of beta-cell function, study duration and length of follow-up, attrition rate during follow-up, method of randomisation and blinding, study design, funding source and conflict of interest.
Risk of bias assessment
Individual randomised controlled trials were assessed for risk of bias using the RevMan 5.3 software, which is based on the Cochrane Risk of Bias tool. Studies were judged for risk of bias with regard to the following criteria: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias, which included sponsorship bias.
Results
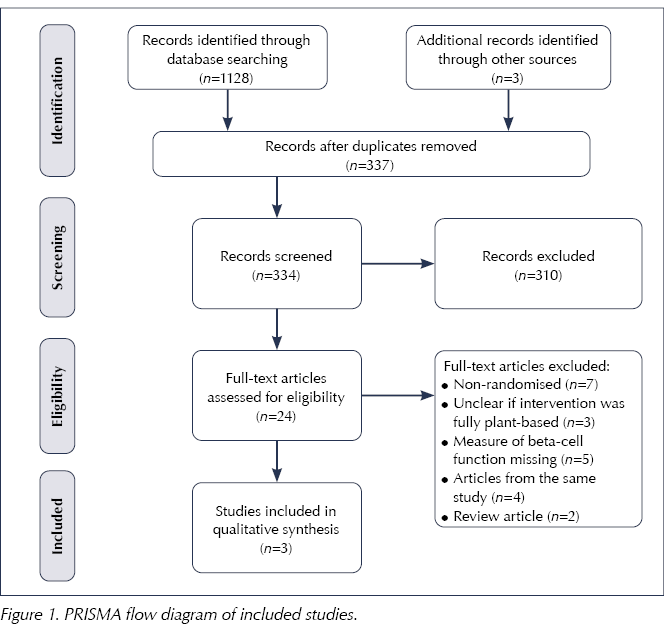
The initial systematic search of the literature yielded 1131 titles and abstracts. Of these, 24 papers were read in full and three have been included in this systematic review (Figure 1).
A publication by Lim et al (2011) was excluded from this systematic review upon receipt of clarification via email from the original authors, who confirmed that the intervention diet contained meat protein. The design of this study was non-randomised and there was no control group.
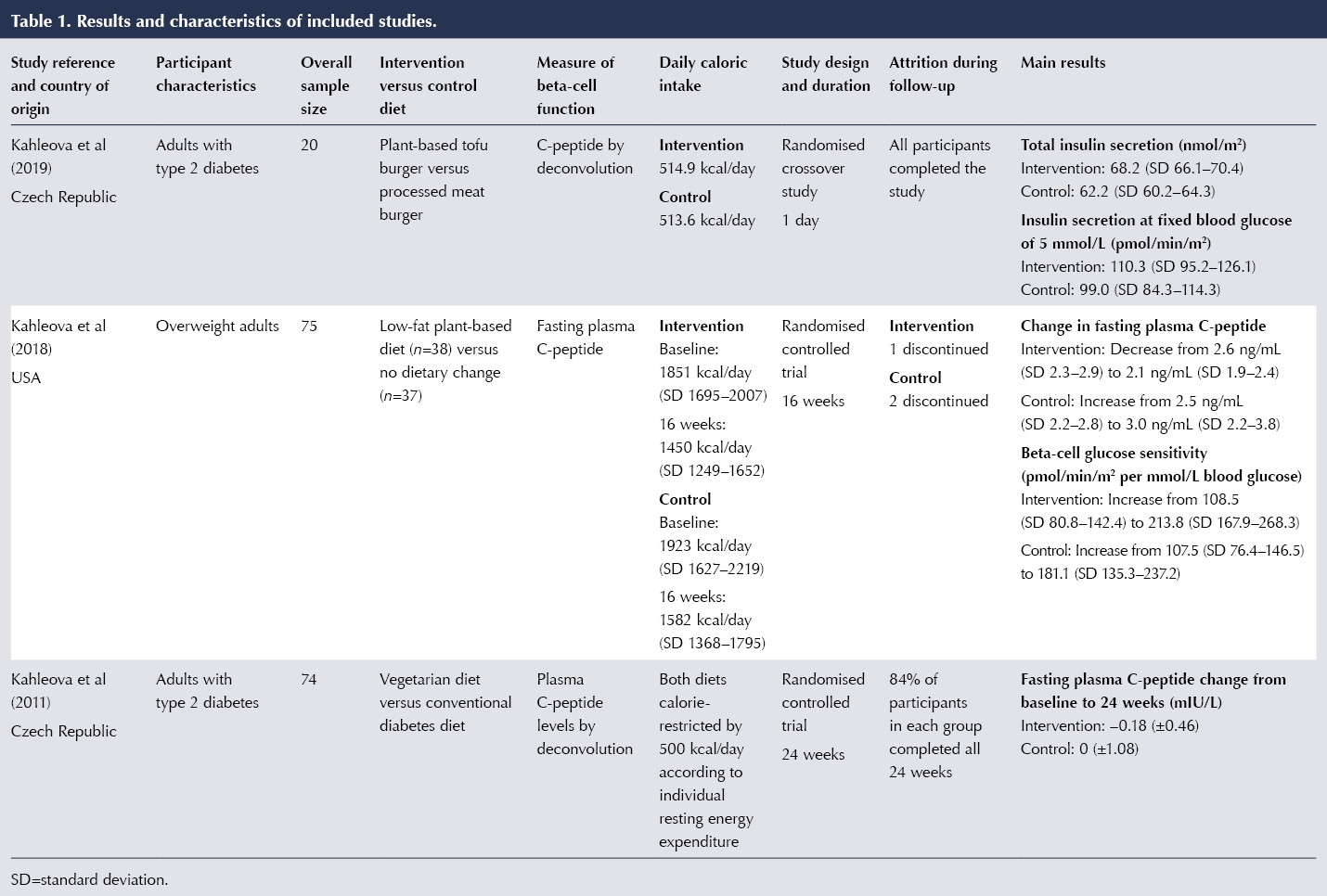
The main results and characteristics of the included studies are summarised in Table 1.
All three of the included trials had the same lead author, and one of the studies only investigated the effect of a single meal on pancreatic beta-cell function, meaning that the intervention period differed significantly between the three trials. Therefore, it was decided that a quantitative synthesis in the form of meta-analysis would not be appropriate.
Among the three studies included, all had a randomised controlled trial design (Kahleova et al, 2011; 2018; 2019). The sample sizes of the individual studies ranged between 20 and 75 participants. The participants comprised adults with type 2 diabetes in two of the trials (Kahleova et al, 2011; 2019) and overweight adults in the other (Kahleova et al, 2018). All three trials controlled for caloric intake, and the two longer-term studies utilised calorie-restricted diets in both groups. Kahleova et al (2011) reduced caloric intakes in both groups by 500 kcal/day based on the measurement of resting energy expenditure of each subject.
The study by Kahleova et al (2019) involved two meal tests with a 1-week washout period between the meals, during which participants were asked to consume their regular diets. The other two trials were 16 and 24 weeks in duration.
Kahleova et al (2019) aimed to investigate if a plant-based meal stimulated postprandial incretin and insulin release. Kahleova et al (2018) aimed to test the effect of a dietary intervention on beta-cell function and insulin resistance in overweight adults with no history of diabetes. The third study hypothesised that a plant-based diet would be more effective in improving insulin resistance than a conventional diet for people with diabetes (Kahleova et al, 2011). Even though the main study objective concerned insulin resistance, it reported parameters relating to beta-cell function, such as fasting plasma C-peptide concentration.
Among people with type 2 diabetes in the trial by Kahleova et al (2011), 43% in the plant-based group were required to reduce their diabetes medication, compared with 5% in the control group (P<0.001). This was done by a study physician in cases of repeat hypoglycaemia.
Kahleova et al (2019) examined the effects of a vegetarian burger versus a meat burger, matched for energy and macronutrient content, on postprandial incretin and insulin secretion. This was a randomised crossover trial that only recruited men with type 2 diabetes (n=20). The study’s primary outcome was the area under the curve for postprandial insulin secretion. The vegetarian meal stimulated increased secretion of immunoreactive insulin (by 30.5%) and C-peptide (by 7.1%) compared with the meat meal (P<0.001 for both comparisons). Ingestion of the vegetarian meal resulted in significantly increased total insulin secretion 68.2 nmol/m2 vs 62.2 nmol/m2). Insulin secretion at 5 mmol/L glucose was 11.4% higher after the vegetarian meal compared with the meat meal (P=0.04). In addition, rate sensitivity increased by 55% (P=0.04), and the potentiation factor by 13.3% (P=0.02) after the vegetarian meal compared with the meat meal.
Kahleova et al (2018) observed a decrease in basal insulin secretion in the intervention group at 16 weeks (treatment effect –54.2 pmol/min/m2). The intervention group also had increased beta-cell glucose sensitivity; however, this did not reach statistical significance compared with the control group. The researchers found a dose–response increase in insulin secretion as a function of plasma glucose concentrations in the intervention group compared with controls. Changes in beta-cell function correlated negatively with changes in BMI (r=–0.25; P=0.04), but not with changes in visceral fat.
In Kahleova et al (2011), the intervention diet consisted of vegetables, fruits, grains, legumes and nuts, and a single portion of low-fat yogurt per day was permitted. The control group was administered a conventional diet for diabetes based on the guidelines of the Diabetes and Nutrition Study Group and the European Association for the Study of Diabetes. Fasting plasma C-peptide fell by 0.18±0.46 mIU/L in the intervention group at 24 weeks. HbA1c levels in the intervention group decreased significantly from baseline to 24 weeks (reductions of 7.1 mmol/mol vs 2.3 mmol/mol). The intervention group lost significantly more weight than the control group (6.2 kg vs 3.2 kg).
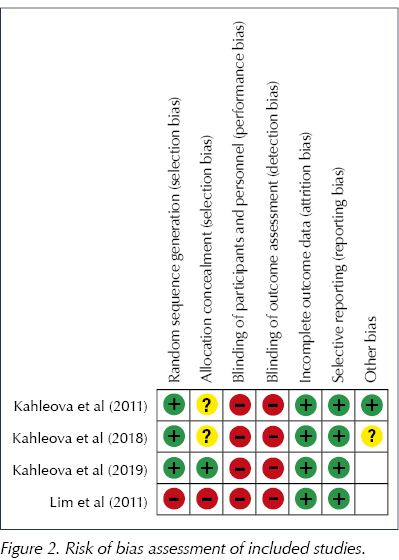
The risk of bias analysis revealed that all included trials were at a high risk of bias, mainly owing to the lack of blinding in the trials. Neither researchers nor participants were blinded to the intervention in any of the trials, as shown in Figure 2. All of the trials used randomisation; however, allocation concealment was unclear in two of the trials (Kahleova et al, 2011; 2018).
Discussion
To the best of our knowledge, this is the first systematic review investigating the effect of plant-based diets on pancreatic beta-cells. The review included three randomised controlled trials. Owing to significant heterogeneity between the trials, a meta-analysis has not been conducted.
All included studies reported beta-cell function as measured by C-peptide concentration. C-peptide is secreted by pancreatic beta-cells together with insulin. It has been widely used as a reliable measure of beta-cell function due to its lower degradation rate than insulin (Acosta-Montaño et al, 2018).
This systematic review suggests that glycaemic control and beta-cell function, as measured by insulin secretion, can be improved by a plant-based diet. Postprandial insulin synthesis was significantly higher after a vegetarian burger compared with a meat burger (Kahleova et al, 2019). In the 24-week trial by Kahleova et al (2011), 43% of participants in the intervention group were required to reduce their diabetes medication. It is not clear from the data whether this was due to improved insulin sensitivity or production, or both; nonetheless, this was a clinically significant outcome.
Normal concentrations of C-peptide in people without diabetes are between 0.5 and 2.7 ng/mL. Therefore, the reduction of 0.5 ng/mL in fasting levels in Kahleova et al (2018) was a clinically significant one, particularly when compared with the 0.5 ng/mL rise (an indirect indication of hyperinsulinaemia) seen in the control group.
The reduction in HbA1c at 24 weeks observed by Kahleova et al (2011) was also clinically significant compared with the control group. HbA1c levels and a reduction in diabetes medications may not be a direct measure of beta-cell function, but they are certainly a measure of glycaemia and diabetes management, and the plant-based diet groups experienced improvements in both measures in this trial.
Strengths and limitations
The main strength of this systematic review is that the trials included were all randomised controlled trials and we did not rely on observational data from cohort or case–control studies.
An important limitation is the lack of blinding in the design of the three studies. Although we intended to include non-randomised trial designs, the exclusion of the study by Lim et al (2011) meant that only randomised controlled trials have been included. A stricter selection was not possible due to the limited number of studies.
Another limitation of our systematic review is the lack of quantitative analysis. All three trials were led by the same researcher, which raises questions about the possible systematic bias that may have arisen. In addition, there was significant heterogeneity between the three trials in terms of study duration. The sample sizes across the included clinical trials were relatively small, thus decreasing the power of the individual studies. Therefore, it was decided that a meta-analysis would not be appropriate, and that a quantitative synthesis of the results to generate a pooled effect size was not possible.
Only one of the three included trials aimed to investigate the effects of plant-based diets on beta-cell function as the primary outcome. This is of importance as multiple outcomes within a study may increase the likelihood that the findings are a result of chance.
Consideration of potential confounders
A significant confounder in both of the longer trials (Kahleova et al, 2011; 2018) was caloric restriction; however, both intervention and control groups were required to consume a calorically restricted diet in both of the trials. Calorie restriction on its own has been shown to improve beta-cell function as reported in the scientific literature. Lim et al (2011) showed that, within a week, a 600 kcal/day diet normalised beta-cell function, as measured by fasting plasma C-peptide and plasma insulin levels, as well as blood glucose levels, in people with type 2 diabetes. However, it seems there may be additional benefits gained from plant-based diets independent of caloric restriction, based on the evidence from this systematic review as well epidemiological research by Tonstad et al (2009), who reported that vegetarian diets offered protection against risk of type 2 diabetes, and that this association remained significant after adjusting for BMI.
Findings in the context of other research
Beta-cells have low glycogen stores as over 90% of the glucose that enter them is oxidised via glycolysis. This results in dependence on fatty acids for energy under fasting conditions, thus reducing intracellular lipid stores (Acosta-Montaño et al, 2018). This partly explains why beta-cell function seems to improve in response to calorie restriction or fasting. Saturated fats, found predominantly in animal fat, have been shown to reduce pancreatic beta-cell proliferation and induce lipoapoptosis, which gradually results in loss of beta-cell mass (Maedler et al, 2001). None of the included studies investigated beta-cell mass loss or examined the relationship between saturated fat intake and beta-cell mass.
Pancreatic beta-cells are particularly vulnerable to oxidative stress created by glucotoxicity, lipotoxicity or hypoxia, due to the lack of antioxidant enzymes present in the cells (Wang et al, 2017). The possible molecular mechanisms by which plant-based diets exert their effects on pancreatic beta-cells may be numerous; however, plant compounds such as flavonoids have been shown to preserve both the function and the survival of beta-cells through their antioxidant and anti-inflammatory properties (Ghorbani et al, 2019).
Vegan or vegetarian diets can easily be high in processed carbohydrates and fats; therefore, the quality of plant-based diets varies greatly. Although Kahleova et al (2011) did prepare and provide the meals for participants, the specific quantities and ingredients of the meals are not reported. Kahleova et al (2019) did not investigate habitual intake as the intervention consisted of a single meal. It would be useful for future trials to focus on whole-food plant-based diets and report specific quantities of nutrients, minerals and vitamins consumed to allow for a comprehensive analysis.
Attrition rates in the intervention groups across the two longer studies were lower than in the control groups, suggesting that plant-based diets have high adherence rates and are sustainable. These factors are especially important as the success of dietary strategies for weight loss and diabetes management relies on how easy it is for individuals to remain on the diet over the long term. The longest-duration study, of 24 weeks, only included people who were willing to make dietary and lifestyle changes (Kahleova et al, 2011), which decreases the generalisability of the findings, as not all people with obesity or diabetes are willing to make such changes.
Restrictive diets may not be appropriate for all, and special considerations must be paid to ensure the nutritional adequacy of the diet. Of special concern are vitamin B12 and vitamin D, which have been shown to be deficient in vegan populations (Sakkas et al, 2020); therefore, plant-based dietary interventions should utilise supplements, or participants should be encouraged to consume fortified food items. Despite the restrictive nature of exclusively plant-based diets, the reviewed clinical trials showed high compliance rates.
Study quality and implications for future research
All trials were deemed to be at a high risk of bias as per the Cochrane Risk of Bias handbook (Higgins and Thomas, 2019), even though all had used adequate randomisation and the baseline characteristics of participants were comparable across studies. Based on this systematic review, further evidence is required in the form of randomised controlled trials with standardised measurements of beta-cell function and large sample sizes to determine whether plant-based diets can improve or restore beta-cell function. Unfortunately, blinding of participants and/or researchers in these trials will most likely be impractical or not possible.
Conclusion
This systematic review aimed to investigate whether a plant-based diet can positively affect pancreatic beta-cell function. Due to the limited number of clinical trials, we are unable to report a firm conclusion. A number of parameters relating to blood glucose management showed significant improvements in the plant-based groups from the included trials; however, larger randomised controlled trials with uniform measurements of beta-cell function (pre- and postprandial plasma C-peptide concentration over a period of time) would be beneficial to allow for quantitative analysis of pooled results.





Helping homeless adults to overcome the challenges of managing their condition.
16 Apr 2024