Lifestyle
The high prevalence of cardiovascular disease (CVD) in people with diabetic kidney disease (DKD) reinforces the importance of a healthy diet, regular exercise, avoiding smoking and weight management (NICE, 2021; American Diabetes Association [ADA], 2025). Restriction of dietary salt can help manage hypertension and reduce cardiovascular (CV) risk. Moderation of alcohol consumption should be encouraged.
In advanced stages of DKD, low potassium and low phosphate diets may be required, though referral to specialist services is likely to have taken place at this point.
Treatments providing cardiorenal protection
Renin–angiotensin system inhibitors
ACE inhibitors (ACEis) and angiotensin receptor blockers (ARBs) inhibit the renin–angiotensin system (RAS). They are key agents in DKD, as they provide protection against CV events and attenuate the progress of renal disease once albuminuria has developed (ACE Inhibitors in Diabetic Nephropathy Trialist Group, 2001; Lewis et al, 2001; Catala-López et al, 2016). The latter is achieved by inducing glomerular efferent arteriole vasodilation, thereby reducing intraglomerular pressure and protecting the glomerular basement membrane.
ACEis/ARBs are generally first-choice anti-hypertensive medications in people with diabetes but, even in the absence of hypertension, they should be prescribed in albuminuric type 1 diabetes or type 2 diabetes because their cardiorenal benefits are evident beyond those attributable to blood pressure (BP; Heart Outcomes Prevention Evaluation (HOPE) Study Investigators, 2000; Brenner et al, 2001; NICE, 2021). In the absence of albuminuria, the renoprotective properties of ACEis/ARBs are less clear (ADA, 2025). NICE advises that the dose of ACEi or ARB should be titrated up gradually to the highest licensed dose that is tolerated if albumin-to-creatinine ratio (ACR) is ≥3 mg/mmol in adults with type 1 or type 2 diabetes (NICE, 2021).
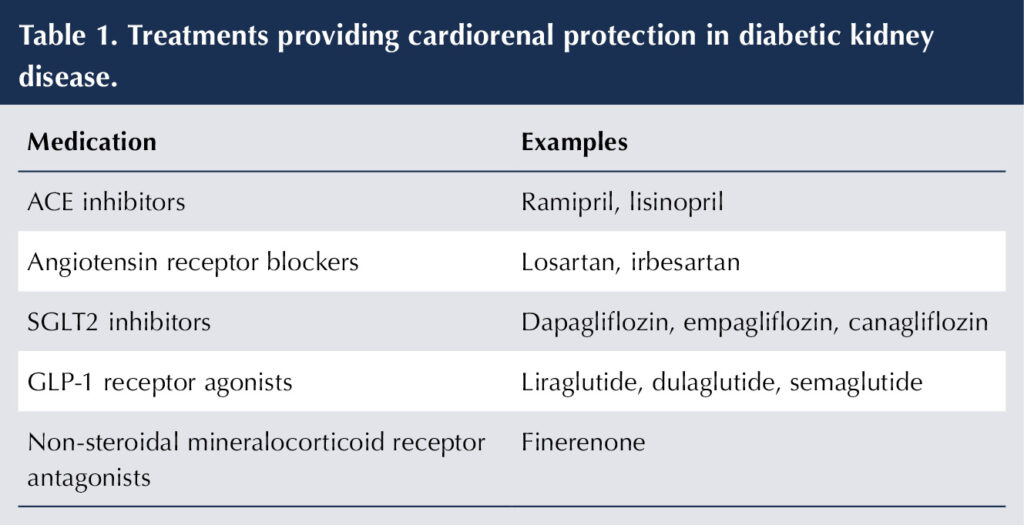
Table 1 summarises the treatments that provide cardiorenal protection, while Box 1 highlights some practical points on using RAS inhibitors.

SGLT2 inhibitors
In people with type 2 diabetes, cardiovascular outcome trials (CVOTs) with empagliflozin, canagliflozin and dapagliflozin have established that use of these SGLT2 inhibitors reduces the risk of CV events (quantified as a composite of non-fatal myocardial infarction, non-fatal stroke and CV death) versus placebo in:
- Established atherosclerotic CV disease (ASCVD; angina, myocardial infarction, ischaemic stroke or transient ischaemic stroke [TIA], peripheral vascular disease) – secondary prevention.
- Those at high risk of CV events – primary prevention (Zinman et al, 2015; Neal et al, 2017; Wiviott et al, 2019).
The CVOTs with canagliflozin, dapagliflozin and empagliflozin also identified improved secondary composite renal outcomes, while the ertugliflozin CVOT showed a positive trend that just fell short of significance (Cannon et al, 2020).
Subsequently, double-blind randomised placebo-controlled trials in subjects with type 2 diabetes and CKD with a primary renal outcome (typically a composite of renal function decline, end-stage kidney disease [ESKD] and death from a renal or CV cause) have been performed. The trials with canagliflozin, dapagliflozin and empagliflozin demonstrated improved renal outcomes and reduced CV mortality irrespective of baseline eGFR (Perkovic et al, 2019; Heerspink et al, 2020; Herrington et al, 2023). Importantly, most people in these studies were already established on ACEi/ARB therapy, indicating that benefits from these agents and the SGLT2is are additive. The dapagliflozin and empagliflozin trial populations included people without type 2 diabetes and, significantly, they received similar benefits to those with type 2 diabetes.
NICE recommends that people with type 2 diabetes who have CKD should, in addition to receiving treatment with an ACEi or ARB:
- Be offered an SGLT2i if uACR >30 mg/mmol.
- Be considered for an SGLT2i if ACR is between 3 and 30 mg/mmol (NICE, 2022a).
The specific NICE recommendations for use of dapagliflozin and empagliflozin in CKD (with or without type 2 diabetes), including eGFR and uACR values at initiation, are available (NICE, 2022b; 2023c). The ADA and Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend using SGLT2is with demonstrated benefit in reducing CKD progression and CV events in individuals who have type 2 diabetes with an eGFR of ≥20 mL/min/1.73 m2 (KDIGO, 2022; ADA, 2025) and the ABCD–UKKA guideline in individuals with an eGFR of ≥15 mL/min/1.73 m2 (Dasgupta et al, 2024).
The SGLT2is protect the glomerular basement membrane by reducing intraglomerular pressure via vasoconstriction of the glomerular afferent arteriole. This accounts for the small reduction in eGFR seen on initiation. Subsequently eGFR is stabilised and albuminuria reduced.
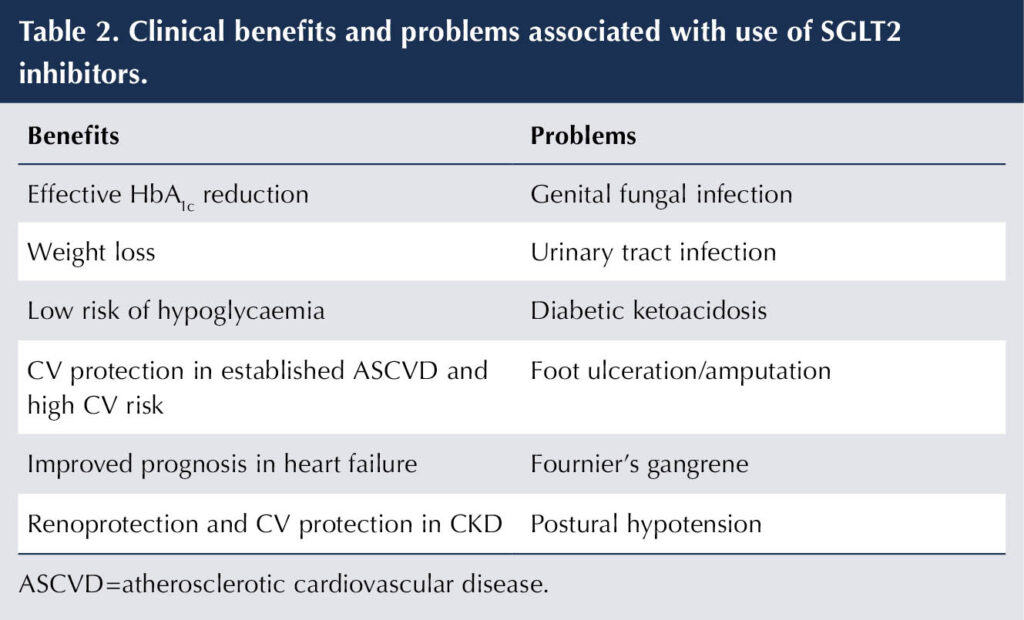
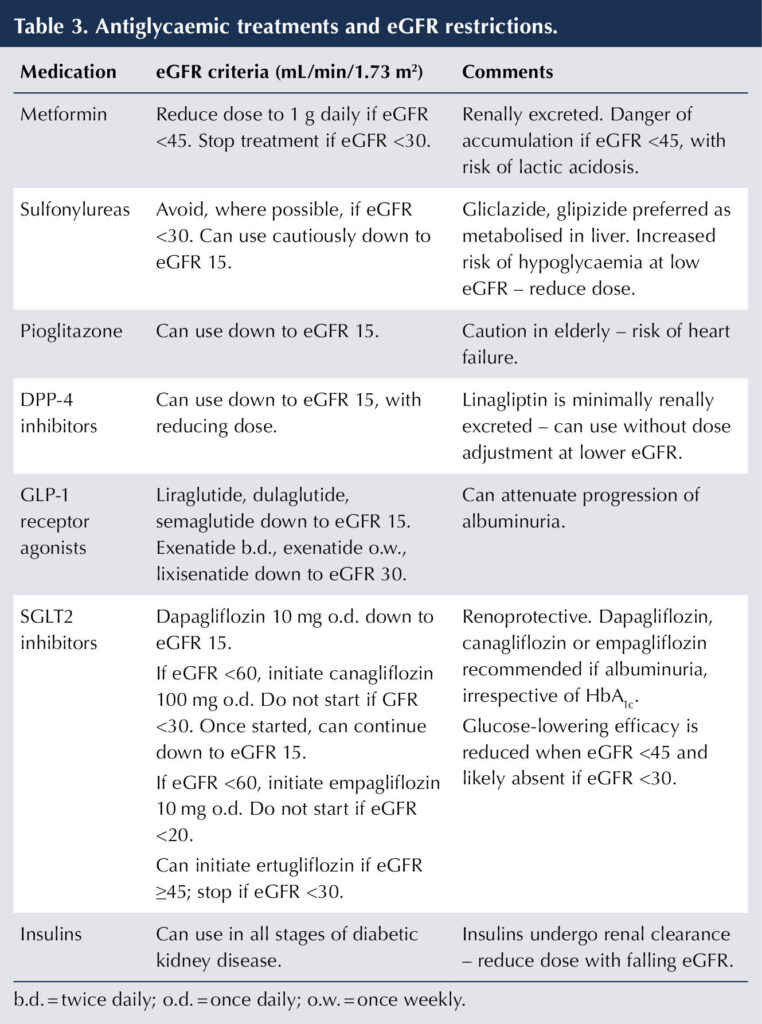
SGLT2 inhibitors are, therefore, established as key agents in DKD to reduce CKD progression and CV events. Table 2 shows the clinical benefits and problems associated with this class of medication, while Table 3 outlines the licensed eGFR ranges for their use. It should be noted that dapagliflozin and empagliflozin improve prognosis in heart failure and are licensed for this purpose whether or not type 2 diabetes is present (Electronic Medicines Compendium [emc], 2024; 2025a).
GLP-1 receptor agonists
CVOTs with liraglutide, dulaglutide and semaglutide demonstrate improved CV outcomes, compared to placebo, in people with type 2 diabetes with established ASCVD or with risk factors for this, including DKD (Marso et al, 2016a; 2016b; Gerstein et al, 2019).
The secondary renal outcomes from CVOTs with GLP-1 receptor agonists (RAs) consistently show benefit in DKD, largely through restricting the progression of albuminuria (Kristensen et al, 2019). More recently, the FLOW trial with semaglutide in people with type 2 diabetes and CKD – the first randomised controlled trial with a dedicated renal outcome for GLP-1 RAs – was reported (Perkovic et al, 2024). A reduction in the composite primary renal outcome was found with semaglutide compared to placebo. A reduction in both uACR and rate of decline of eGFR was identified. Secondary outcomes also favouring semaglutide included a reduction in CV events and all-cause mortality versus placebo.
GLP-1 RAs are an important consideration for use in type 2 diabetes where CKD has been identified, as they bring both CV and renal benefits (ADA, 2025). Table 3 outlines the licensed eGFR ranges of some key GLP-1 RAs.
In a post hoc analysis of the SURPASS-4 trial in people with type 2 diabetes and high CV risk, the first-in-class dual GIP/GLP-1 RA tirzepatide was found to reduce albuminuria and slow the rate of eGFR decline compared to insulin glargine (Heerspink et al, 2022).
Finerenone
Finerenone is a non-steroidal mineralocorticoid receptor antagonist (contrast with spironolactone, a steroidal mineralocorticoid RA) that has been shown to reduce the risk of CKD progression (Bakris et al, 2020) and improve CV outcomes (Pitt et al, 2022) compared to placebo in randomised controlled trials in subjects with type 2 diabetes and albuminuric CKD.
Finerenone now holds a licence for treating people with type 2 diabetes and CKD (emc, 2023b). NICE has recommended it as an option for treating CKD with eGFR in the range 25–60 mL/min/1.73 m2 and albuminuria in people with type 2 diabetes to provide renal and CV protection. Finerenone can be added to standard care for people with type 2 diabetes and CKD, including the highest tolerated dose of ACEi or ARB and an SGLT2i (NICE, 2023d). Finerenone is a potassium-retaining medication, so monitoring for hyperkalaemia is necessary, particularly when it is used alongside an ACEi/ARB.
Glycaemic control in DKD
The importance of effective glycaemic control for prevention of microvascular problems and, in particular, DKD have been demonstrated for both type 1 and type 2 diabetes (DCCT Research Group, 1993; UKPDS, 1998a). Once DKD is established, good glycaemic control can attenuate deterioration and progression to ESKD (Skupien et al, 2014). The caveat to aiming for intensive glycaemic control is that individual circumstances should be assessed in setting HbA1c targets to take account of comorbidities, risk of hypoglycaemia, concurrent medication, social circumstances and patient wishes (NICE, 2022a; ADA, 2025).
Because of their cardiorenal benefits, SGLT2is should be a first-line choice for managing hyperglycaemia alongside metformin in DKD. It is important to recognise that the glucose-lowering capability of SGLT2is falls with declining eGFR (notably when <45 mL/min/1.73 m2), although their cardiac and renal benefits are maintained, so they should be utilised in DKD even if the prevailing eGFR predicts that they will contribute little to glycaemic control.
GLP-1 RAs should also be prioritised if further glycaemic control is required in the individual with DKD because they again offer cardiorenal benefits. As indicated above, the strongest evidence lies with semaglutide.
Table 3 indicates the renal function restrictions for treatments for hyperglycaemia (BNF, 2025; emc, 2023a; 2024; 2025a; 2025b). The temporary withdrawal of medication in the situation of acute illness to avoid complications, such as AKI, is covered in Part 1 (Morris, 2025).
Addressing cardiovascular risk factors
Hypertension
In addition to preventing onset of CKD, BP control is a crucial intervention in established DKD to reduce both the risk of CV events and decline in renal function (UKPDS, 1998b). NICE guidance advises clinic BP targets of <140/90 mmHg if uACR <70 mg/mmol, and <130/80 mmHg if ACR is ≥70 mg/mmol for people with either type 1 or type 2 diabetes (NICE, 2021; NICE, 2022c). Blood pressure targets are the same for individuals over the age of 80 years with DKD (NICE, 2023e). If ambulatory or home BP monitoring are being used to assess BP, then targets are 5 mmHg lower than the corresponding clinic readings.
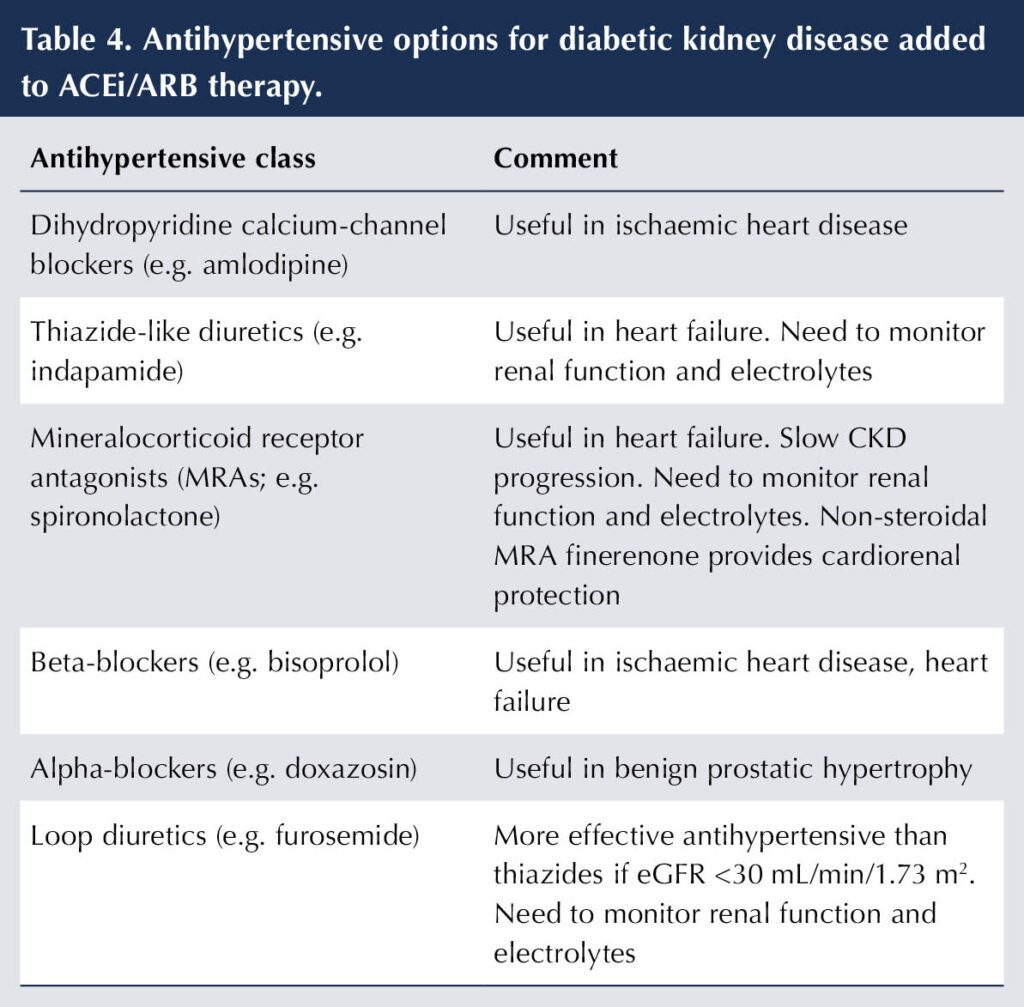
The cardiorenal benefits associated with ACEis and ARBs in DKD (see previously) place them as first-line antihypertensive agents. Additional antihypertensives may be added, as necessary, to achieve BP target (see Table 4).
Dyslipidaemia
As CKD is such a high-risk condition for CVD, NICE recommends the following (NICE, 2023f):
- Offering atorvastatin 20 mg once daily to all people with CKD for primary or secondary prevention of CVD, including those with diabetes (without recourse to QRISK).
- Target of a 40% reduction in non-HDL cholesterol for primary prevention of CVD.
- Target of a non-HDL cholesterol of ≤2.6 mmol/L or an LDL-cholesterol of ≤2.0 mmol/L for secondary prevention of CVD.
- If the lipid target is not achieved and eGFR is 30 mL/min/1.73 m2, the dose of atorvastatin can be increased up to a maximum of 80 mg once daily.
- If eGFR <30 mL/min/1.73 m2, then discuss use of higher doses of atorvastatin with a renal physician.
An alternative, perhaps simpler, lipid target for primary prevention of CVD is the treat-to-target strategy, aiming for a non-HDL cholesterol target of 2.5 mmol/L, as recommended by the Joint British Societies (JBS3, 2014).
If necessary, additional agents, such as ezetimibe and bempedoic acid (caution if eGFR <30 mL/min/1.73 m2), may be added from primary care (emc, 2025c). Should statins other than atorvastatin be used, then the BNF or SmPC should be consulted with regard to use in CKD. Thus, the dose of rosuvastatin should not exceed 20 mg once daily if eGFR is <60 mL/min/1.73 m2 and it should be avoided altogether if eGFR is <30 mL/min/1.73 m2 (BNF, 2025).
Antiplatelet medication
Antiplatelet medication, such as aspirin or clopidogrel, is not routinely recommended for primary prevention of CV disease in DKD, but should be used for secondary prevention – angina, peripheral vascular disease, previous myocardial infarction, stroke or TIA (NICE, 2021; KDIGO, 2022).
Conclusion
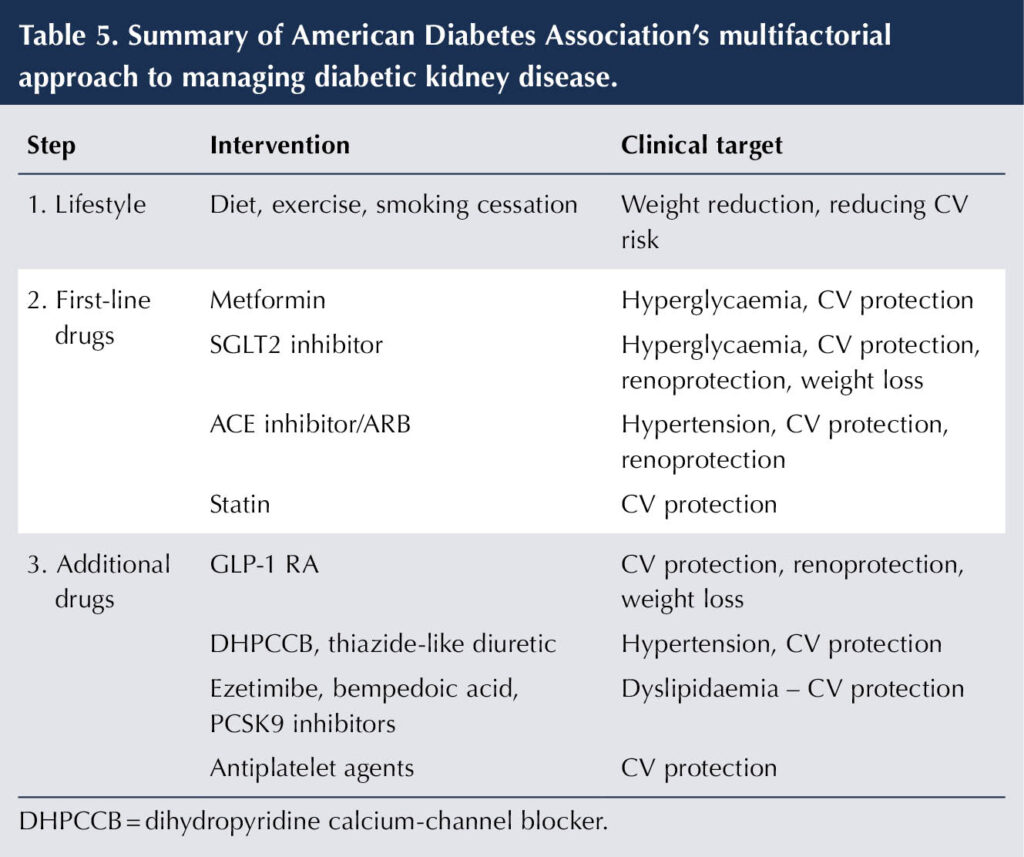
Managing DKD requires a multifaceted approach, starting with lifestyle advice, addressing the CV risk factors of hypertension and dyslipidaemia, treating hyperglycaemia, and harnessing the cardiorenal protective properties of specific drugs. Both the ADA (2025) and the Association of British Clinical Diabetologists/UK Kidney Association (ABCD-UKKA) (Dasgupta et al, 2024) have issued recommendations for managing CKD in type 2 diabetes that use a stepwise approach to interventions that cover the multiple factors that impact on DKD. Table 5 summarises the ADA strategy.






Developments that will impact your practice.
26 Jun 2025