The population is ageing, with 11.8 million people aged 65 years or older and 1.6 million aged over 85 now living in the UK (Age UK, 2018). As the prevalence of type 2 diabetes and dementia is greater in older people, it is not surprising that both conditions are significantly increasing in numbers, with over 850 000 people with dementia (Dementia UK, 2017a) and almost 3.7 million people with diabetes (Diabetes UK, 2018a) in the UK. In addition, people with type 1 diabetes are now living with the condition into old age. Both diabetes and dementia are progressive, complex long-term conditions that consume a considerable amount of health and social care resources. As the population gets older, nurses working in most areas of healthcare are likely to see more people living with the combination of the two conditions. By 2025, the number of people with dementia is predicted to rise to over 1 million, and to 2 million by 2050 (Prince et al, 2014), and the number of people with diabetes is predicted to rise to over 5 million by 2025 (Diabetes UK, 2018a).
Dementia is a progressive, irreversible condition of the brain which causes widespread impairment of mental function. Presentation varies between individuals but usually involves a range of cognitive and behavioural signs and symptoms, including memory loss, problems with reasoning and communication, changes in personality and a reduction in ability to carry out daily activities of living (NICE, 2018). As the dementia progresses, it leads to restlessness, wandering, eating problems, incontinence, delusions, hallucinations, mobility difficulties leading to falls and fractures, and increasing dependence on others. The most common types of dementia are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, frontotemporal dementia and mixed dementia, although there are over 200 different types (Dementia UK, 2017a). One in 14 people over the age of 65 years, and one in six in those aged over 80 years, has dementia (Prince et al, 2014). However, more than 42000 people aged under 65 years also have the condition. It is more common in women than in men.
Diagnosis of dementia
The diagnosis of dementia is made after taking a history of signs and symptoms, a cognitive and mental state examination, a physical examination including blood tests, and a medication review (to exclude any drugs that may affect cognitive functioning). A validated brief structured cognitive instrument should be used to assess mental function, such as the 10-point Cognitive Screener (10-CS), the six-item Cognitive Impairment Test (6CIT), the Memory Impairment Screen (MIS) or the Mini-Cog (NICE, 2018). These assess abilities in attention and concentration, orientation, short- and long-term memory, praxis, and language function. Factors that may affect the results of these tests, such as prior educational level, usual spoken language, presence of any psychiatric illness and sensory impairments, also need to be considered. Examination of brain structure and vascular changes by MRI or CT scans may be needed to exclude other cerebral disorders, such as a tumour.
Diabetes and dementia
There is a close association between type 2 diabetes and dementia, in particular Alzheimer’s disease and vascular dementia, with type 2 diabetes associated with a 60% increase in risk of all-cause dementia (Gudala et al, 2013). As type 2 diabetes is a known risk factor for cardiovascular and cerebrovascular disease, this may explain the association with vascular dementia. Insulin has an important role in the healthy functioning of the central nervous system. The brain is rich in insulin receptors but the hyperinsulinaemia associated with insulin resistance (a key feature of type 2 diabetes) reduces the sensitivity of these insulin receptors and so reduces the passage of insulin into the brain (Cholerton et al, 2016). There is a 56% increased risk of developing Alzheimer’s disease in individuals with type 2 diabetes (van de Vorst et al, 2016) and, conversely, people with Alzheimer’s disease have an increased risk of developing type 2 diabetes (35% vs 18% of people without dementia) and impaired glucose tolerance (46% vs 24%; Janson et al, 2004). The rate of cognitive decline in older people with type 2 diabetes is double that in those without diabetes (Cholerton et al, 2016). In addition, slowing down of general cognitive function (which is a marker for accelerated brain ageing and dementia risk) is related to type 2 diabetes in middle-aged and older adults.
Dementia care in the UK
An NHS mandate published by the Department of Health (DH) in 2012, updated in 2015, aimed to ensure that the diagnosis, treatment and care of people with dementia in England would be the best in the world (DH, 2015). This would be achieved by improving early diagnosis through raising awareness, access to memory assessment and diagnostic clinics, access to the right information at the right time, and improving the experience for people seeking help for memory problems.
There have been three Quality Standard documents produced by NICE relating to dementia:
- QS1: Dementia quality standards (NICE, 2010)
- QS30: Supporting people to live well with dementia (NICE, 2013a)
- QS50: Mental wellbeing of older people in care homes (NICE, 2013b)
The statements that comprise these Quality Standards are listed in Table 1.
Early diagnosis of dementia and diabetes is important
The development of dementia in people who already have diabetes will lead to difficulties with self-management and adherence to medication, including safe self-administration of insulin injections. If the diagnosis of dementia is delayed, individuals may appear to be non-concordant or disengaged with their diabetes care, or they may present with acute complications such as severe hypoglycaemia or diabetic ketoacidosis. Having both conditions may mean agreeing higher targets for blood glucose and blood pressure to keep the individual safe, and working with the person and their family to make sensible decisions about the future.
Diagnosing diabetes early in people who already have dementia will ensure they receive regular review and management of symptoms. If required, medications can be introduced to relieve the symptoms of hyperglycaemia, which will improve quality of life (e.g. reduce tiredness, frequency of urination and thirst) and avoid hospital admissions for hyperglycaemia.
Implications for people with dementia who develop diabetes
If people with dementia are unable to recognise or communicate symptoms of diabetes, the diagnosis may be missed or delayed. Worsening confusion from dehydration and tiredness caused by hyperglycaemia-induced diuresis may be attributed to the progression of dementia. The frequent need to urinate increases the risk of falls, or the development of incontinence if the person forgets where the toilet is. Significant changes in the diet or the need for blood glucose monitoring or insulin injections may cause distress if the person is unable to understand they have diabetes. If they have neuropathic pain but are unable communicate this, it may result in wandering, rocking movements and crying.
Implications for people with existing diabetes who develop dementia
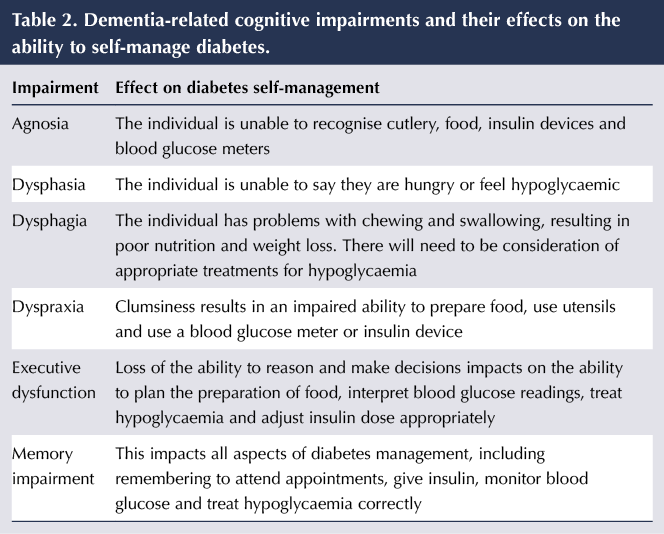
The loss of short-term memory increases the risk of hypoglycaemia in those who are treated with sulfonylureas, meglitinides and insulin if they forget to eat regularly or forget that they have taken medication and take it again. Conversely, poor memory increases the risk of hyperglycaemia if they miss medication, have difficulty injecting insulin correctly or forget they have eaten and eat again. They may be unable to make decisions about their blood glucose results, such as adjusting insulin doses appropriately or treating hypoglycaemia. Dementia causes a number of abnormalities which impact on the person’s ability to manage their diabetes; these are listed in Table 2.
If someone has managed their diabetes for many years, becoming dependent on others to do so can result in a real sense of loss, as well as frustration if they are not permitted to continue with aspects of care they are still able to manage. Partners can also become distressed if their loved one’s diabetes management is taken over by carers and altered from a long-established routine. The relaxing of diet and allowing a higher range of blood glucose levels, therefore, need to be discussed sensitively with the individual and his or her family.
Management of diabetes in the individual with dementia
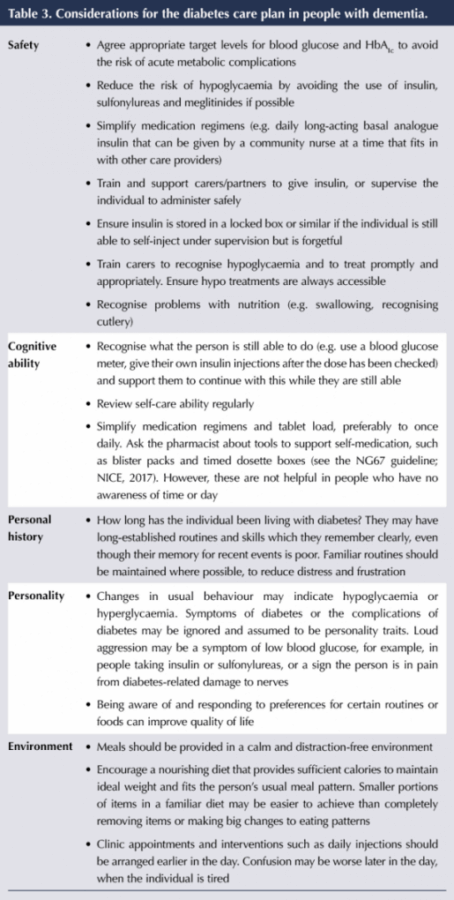
The focus of management for people with both diabetes and dementia is safety (particularly the avoidance of hypoglycaemia) and simplicity, but individualised to accommodate the ability to self-care. Personalised care plans should be regularly reviewed and updated due to the progressive nature of diabetes and dementia (“regularly” will depend on the stage and rate of progression of the dementia). They are useful to share with other people involved in the individual’s care, such as hospital staff, agency staff and dentists. Table 3 suggests some diabetes considerations for the care plan.
The International Position Statement on the Management of Frailty in Diabetes Mellitus (Sinclair et al, 2017) recommends an HbA1c target for mild to moderate frailty as 53–64 mmol/mol (7.0–8.0%) and for severe frailty as 59–69 mmol/mol (7.5–8.5%). Frailty is defined as being dependent, with multiple comorbidities, dementia and living in a care home. These targets take into account the likely benefits of tight glycaemic control versus the risks to the individual, their vulnerability to hypoglycaemia, their ability to self-manage, the presence of other pathologies, their cognitive status and their life expectancy.
Care/support plans should record the preferences of the person with dementia while they still have the mental capacity to make decisions for themselves (DH, 2005), but other documents may be needed giving specific details about their future care (NICE, 2018). These include Advance Statements (a written document signed by the individual while they still have capacity, stating what is to be done if they lose capacity to decide or communicate), an Advance Decision to Refuse Treatment statement, and Preferred Place of Care (stating future care plans and the place where the individual would like to die). Lasting Power of Attorney states who they want to make certain decisions for them if they are unable to do it themselves.
Communication
People with dementia may find it difficult to pronounce words, find the right words to express themselves, concentrate on what is being said to them or remember what has been said. This may cause anxiety and frustration, especially if they are aware that the instructions or information being given about their diabetes is important. Dementia UK (2017b) gives some useful tips on communicating with people with dementia (Box 1).
Changes in behaviour, increasing agitation and anxiety, worsening confusion and a feeling of being in the wrong place is more common at dusk, a phenomenon described as “sundowning” by Dementia UK (2017c). Tiredness, thirst, hunger or pain may be the cause of this. Risk of hypoglycaemia may be more pronounced as a result, due to pacing, shouting and agitation, for example. Concordance with medication or blood glucose monitoring at this time of the day may be challenging. It may be difficult to distinguish between sundowning behaviour and the signs of hypoglycaemia. Arranging clinic appointments or organising medications to be taken earlier in the day may be helpful in this situation.
End-of-life care
As dementia progresses, there is a slow decline in health status, a decrease in appetite and nutritional intake, reduced ability to follow the usual diabetes regimen, increased risk of frailty and vulnerability to infections. Becoming bed-bound or developing urinary and faecal incontinence are signs of advanced dementia and nearing the end of life (Diabetes UK, 2018b). Further relaxing of clinical targets, minimising invasive monitoring and stopping all but essential medications is a reasonable process, with the focus being on the safety of the individual, avoiding hypoglycaemia and maintaining the best possible quality of life. Insulin dose and frequency of injections may be reduced but should never be completely stopped in someone with type 1 diabetes.
Conclusion
Diabetes and dementia are increasingly common conditions and, as the population ages, the number of people living with both conditions will increase. This has significant implications for social and health care resources. Appropriate use of medications, agreeing appropriate glycaemic targets, comprehensive training of carers and the regular review of care plans that take into account residual self-management skills are some of the aspects of care that diabetes nurses will be involved in when caring for people with both of these conditions.
TREND-UK resources
Much of the material for this article has been taken from the recently revised TREND-UK document for healthcare professionals: Diabetes and dementia: Guidance on practical management. This includes information about both conditions, written particularly for people who may not have specialist knowledge in diabetes. This document and a leaflet for patients, Living with diabetes and dementia, can be downloaded from the TREND-UK website. Registration is free.
The Integrated Career and Competency Framework includes competence standards for those working in residential and nursing homes, and with people approaching the end of life. A significant number of people with both conditions are cared for by staff who do not have specialist diabetes skills. The framework can be used to identify the training needs and assess the competency of carers looking after these vulnerable individuals with very complex needs, from unregistered practitioners to senior managers.






Study provides new clues to why this condition is more aggressive in young children.
14 Nov 2025