
The biotechnology enabling synthesis of large biological molecules within living organisms for use as medicines is now well established. Human insulin and analogue insulins are examples of medicines produced in this manner. When patents expire on these insulins, the opportunity arises for other manufacturers to produce copies of the original insulin molecule, and these are termed biosimilar insulins. Biosimilar insulins are becoming increasingly available and can offer a cost advantage to the NHS.
This article seeks to explain the nature of biological medicines and their production, including how biosimilar insulins can subtly differ from the reference (originator) insulin. The licensing requirements for biosimilar insulins are described, most notably the need to prove bioequivalence to the reference insulin. The advantages and safety concerns of using biosimilar insulins are discussed, along with practical aspects of switching to and initiating a biosimilar insulin. The biosimilar insulins available in the UK are reviewed.
Human insulin
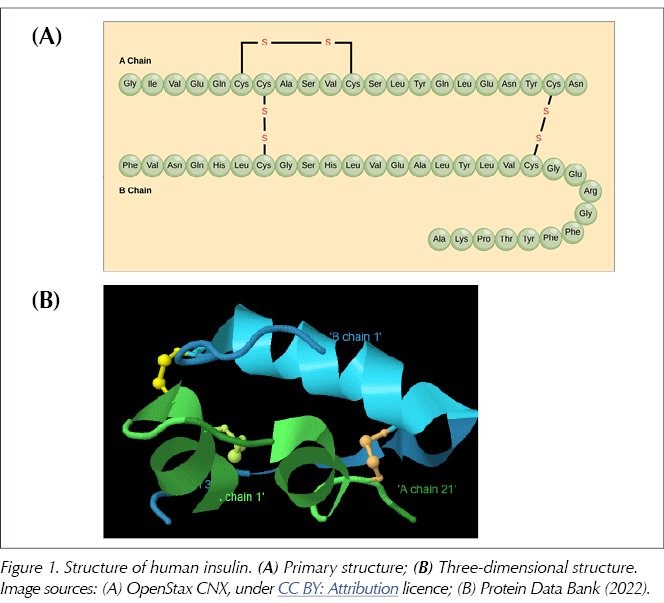
Pancreatic beta-cells first synthesise preproinsulin, an inactive precursor to insulin which is then cleaved by endopeptidase enzymes, first to proinsulin and then to insulin itself, releasing a peptide fragment called C-peptide (Baeshen et al, 2014). The human insulin molecule thus generated has a molecular formula of C257H383N65O77S6 and a molecular weight of just over 5800 daltons. It consists of two peptide chains: an A chain of 21 amino acids and a B chain of 30 amino acids, linked by two disulphide bridges (derived from the amino acid cysteine; see Figure 1). The amino acids themselves are distinguished by the nature of their sidechains and are linked to each other by amide bonds to form the peptide chain (just 20 amino acids are utilised to make all the proteins found in humans). Human insulin may be termed a polypeptide or a small protein (Weiss et al, 2014).
Whilst the primary structure of insulin is defined by the sequence of amino acids, the complex three-dimensional shape which is essential to its function is determined by subtle intramolecular forces (including hydrogen bonding, electrostatic interactions, hydrophobic interactions and disulphide bridges) that generate the so-called secondary and tertiary structures of insulin (Figure 1B; Lumen Learning, 2022).
In fact, the amino acid sequence for insulin is highly conserved between species, such that porcine and bovine insulin differ from human insulin by only one and three amino acids, respectively. These animal insulins retain their function within humans and were, until the advent of biosynthetic human insulin, the medical source of insulin for treating diabetes.
Producing human insulin
Human insulin is now produced by recombinant DNA technology, and this was first achieved for therapeutic use in 1982 (Johnson, 1983). The process involves the insertion of the synthesised human insulin gene (coding for the amino acid sequence of human insulin) into a host organism – typically a bacterium (e.g. Escherichia coli) or a yeast (e.g. Saccharomyces cerevisiae) – which is then multiplied in a fermentation tank (Baeshen et al, 2014). After purification, the precursor insulin is cleaved with proteases in vitro to generate the biologically active insulin for use as a therapeutic medicine (Kuhlmann and Schmidt, 2014). It is important to recognise that human insulin produced in this way within living cells (a biological medicine) may be subtly different from insulin made within the human body (Heinemann and Hompesch, 2014). Prior to the availability of recombinant DNA technology, human insulin was produced by chemical conversion from porcine insulin (by replacing the single differing amino acid with the human amino acid).
Human insulin analogues (both basal and rapid-acting) are also made using recombinant DNA technology in a very similar manner to human insulin (Gotham et al, 2018). Here, the chemically synthesised insulin gene has specific alterations to the human insulin DNA sequence, such that the resultant insulin molecules have small alterations to their primary (amino acid sequence) structure (Baeshen et al, 2014). These structural changes to the molecule, along with further chemical modification where necessary, allow production of insulin analogue molecules that have superior pharmacokinetic and pharmacodynamic profiles compared to human insulin itself.
Biological medicines and biosimilar medicines
Biological medicines are classified as such because they are synthesised within living organisms utilising biotechnology processes such as recombinant DNA technology (NHS England, 2019). All of these molecules have a complex three-dimensional structure that is vital for their function. Other examples of biological medicines apart from human insulin include:
- Human growth hormone.
- Erythropoietin – for anaemia associated with chronic renal failure and cancer chemotherapy.
- Human parathyroid hormone.
- Monoclonal antibodies – notably for use in inflammatory arthritis and inflammatory bowel disease.
A biosimilar medicine is a copy version of an existing approved biological medicine. It is defined as a biological medicine that has no clinically meaningful difference to the originator molecule in terms of quality, safety, efficacy or immunogenicity (European Medicines Agency [EMA], 2014a; NHS England, 2019)
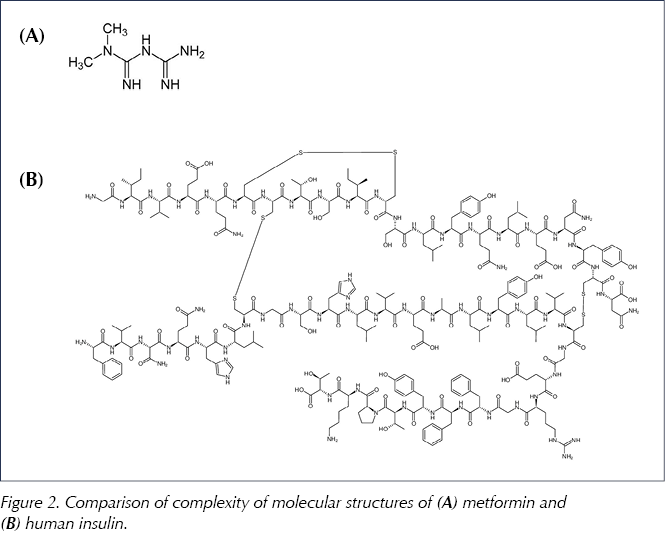
The aim in producing a biosimilar medicine is to reproduce the molecular structure of the originator molecule and hence mirror its biological activity. For small non-biological molecules such as metformin (see Figure 2), this is an achievable target: exact copies of the molecular structure can be synthesised in the laboratory. However, because of the size and complexity of a biological molecule and the accompanying method of production, it is unlikely that a biosimilar molecule will be an exact replication of the originator molecule (EMA, 2017a; Llano et al, 2017).
The option of marketing a biosimilar medicine arises when the patent on the original biological molecule expires. Biosimilar medicines have been authorised by the EMA since 2006, and biosimilars of all the aforementioned biological medicines have been produced. The first biosimilar insulin to be approved in the EU was Abasaglar (insulin glargine, the originator of which was Lantus), in 2014. Further biosimilar insulin products have since followed (see later).
How biosimilar insulins can be different
The intricate manufacturing process of recombinant DNA medicines means that a biosimilar insulin will be subject to different production conditions than the originator insulin. Non-identical strains of the bacteria or yeasts that host the biosynthesis of the insulins, as well as differing incubation processes, are likely to lead to small differences between the biosimilar and its originator.
Furthermore, manufacturing is a batch process, and there may be minor variations in any biological medicine between different batches. This is the case both for biosimilars and for the originator molecules, as both are made within living organisms in a complex manufacturing process. These small differences in structure are termed micro-heterogeneity (EMA, 2017a). Thus, variability may arise not only between the biosimilar and the original product, but also between different batches of the biosimilar, and it is essential that batches are carefully quality-assessed to ensure that safety and efficacy are maintained. The allowed degree of variability of the biosimilar should be the same as that for different batches of the reference medicine. Ultimately, the acceptability of a biosimilar should be judged against the originator molecule (Heinemann and Hompesch, 2014; Kuhlmann and Schmidt, 2014).
Reproducing the primary structure of the insulin (i.e. the sequence of amino acids) in a biosimilar is achieved by selecting the appropriate gene sequence for the insulin. However, chemical modification of the biosimilar insulin may occur during the manufacturing process (including inter-batch variation as described previously). This may, for example, take the form of glycosylation, in which a glucose molecule is attached to the biosimilar, most commonly by bonding to a hydroxyl (-OH) group or to an amino (-NH-) residue on an amino acid side-chain. Another example would be oxidation (the addition of oxygen), to which the sulphide (-SH) groups on the amino acids cysteine and methionine are particularly vulnerable. These chemical changes could potentially lead to subtle alteration of the secondary and tertiary structure (i.e. the folding to a three-dimensional shape) of the biosimilar insulin molecule, which could in turn affect biological function.
Finally, a further influence on a biosimilar’s function might be product-related impurities (Heinemann and Hompesch, 2014; EMA, 2017a).
Establishing bioequivalence of a biosimilar insulin
The small chemical changes in a biosimilar compared to the originator molecule described above could, in theory, affect the efficacy and safety of the biosimilar medicine. There may be an impact on pharmacodynamics (e.g. insulin receptor binding), pharmacokinetics (e.g. bioavailability, metabolism) and immunogenicity. Thus, interchangeability between the biosimilar product and the original molecule cannot be assumed. The physical, chemical and biological properties of the biosimilar must match those of the originator molecule, and quality, safety, efficacy and immunogenicity are tested before use in clinical practice to ensure no clinically meaningful difference between the biosimilar and the reference insulin (Heinemann and Hompesch, 2014; EMA, 2017a).
When a generic version of a small, chemically synthesised molecule is produced, the chemical structure is identical and, thus, interchangeability with the original brand is reasonable to assume. For example, metformin is a small molecule (C4H11N5; molecular weight 129 daltons) with essentially a planar (flat) structure. This is in stark contrast to the much larger and complex, three-dimensional structure of a biological medicine such as insulin (Figure 2). Thus, for insulin, generic substitution is not a valid procedure because the biosimilar can be non-identical. Only after extensive testing and proof of bioequivalence can the biosimilar be considered as a substitute for the original brand (see Box 1). However, it is important to note that this substitution should only ever be undertaken after discussion between clinician and patient, not at the point of dispensing (Heinemann and Hompesch, 2014; EMA, 2017a).

Advantages of biosimilar insulins
When patent protection expires on a medicinal product, other manufacturers can produce this medicine and the competition can lead to a fall in price. The principal benefit from using biosimilar insulins is that they are potentially less expensive than their parent insulins and, thus, costs to the NHS are reduced (Llano et al, 2017). The expensive, large-scale randomised controlled trials needed for the reference insulins are not required for the biosimilar insulin (Diabetes UK, 2019). Rather, the imperative for a biosimilar insulin is to prove bioequivalence (as described above) in comparison studies with the reference insulin.
The cost saving on a biosimilar is not, however, as great in comparison to generic substitution (of a simple non-biological molecule) because the production of a biosimilar insulin still shares the complex processes of production, quality assurance, storage and distribution of the originator insulin. The approval process is also more complicated than for a simple generic drug (Jayagopal et al, 2018; NICE, 2015a). Thus, a biosimilar insulin tends to be around 20% cheaper than the originator insulin.
Once bioequivalence to the reference medicine has been demonstrated for one therapeutic indication, the evidence of safety and efficacy may be extrapolated to other indications for which the reference medicine is already licensed (EMA, 2017a).
Concerns with biosimilar insulins
As detailed previously, the potential variation in structure and function of the biosimilar insulin compared to the original molecule means that bioequivalence in terms of quality, safety, efficacy and immunogenicity need to be rigorously checked.
In addition, the availability of biosimilar insulins increases the risk of prescription and dispensing errors (Jayagopal et al, 2018). To avoid confusion, prescribing by brand name is essential. Generic substitution at the point of dispensing is an accepted procedure for simple organic compounds but is not appropriate for biological medicines, including biosimilar insulins.
There remains the concern over immunogenicity of biosimilar insulins (Jayagopal et al, 2018). The issue here is whether antibodies against the biosimilar insulin may reduce its effectiveness (i.e. glucose-lowering ability). However, the problem of insulin antibodies is generally thought to have been sidelined following the move from animal to human insulin. With respect to analogue insulins, the structural changes are in sections of the molecule that tend not to produce a pronounced immune response (Heinemann and Hompesch, 2014). Thus far, the picture in regard to antibodies that neutralise the function of biosimilar insulins is reassuring; however, ongoing monitoring does need to be continued.
Biosimilar insulins available in the UK
Long-acting insulins
Abasaglar
The first biosimilar insulin to be approved by the EMA was Abasaglar in 2014, and this approval was followed by marketing authorisation in the UK in 2015. Abasaglar is a replication of Lantus, and the two share the identical peptide structure of insulin glargine.
Following preclinical and toxicology studies, pharmacokinetic and pharmacodynamic parameters were demonstrated to be equivalent between Abasaglar and Lantus. Phase III clinical trials were performed in people with type 1 diabetes (ELEMENT 1; Blevins et al, 2015), where individuals were randomised to receive once-daily Abasaglar or Lantus in combination with mealtime insulin lispro, and in people with type 2 diabetes (ELEMENT 2; Rosenstock et al, 2015), where individuals were randomised to receive Abasaglar or Lantus in combination with three or more oral hypoglycaemic agents, having previously taken insulin glargine or been insulin-naïve. Comparable clinical efficacy (non-inferior reduction in HbA1c in a treat-to-target strategy), safety (similar incidence of hypoglycaemia and side-effects) and immunogenicity profile (anti-insulin antibody formation and percentage of antibody binding) were observed between Abasaglar and Lantus in both ELEMENT 1 and ELEMENT 2.
When first marketed, the cost of Abasaglar was 15% less than the parent medication Lantus (Llano et al, 2017); however, the cost of Lantus has subsequently been reduced in 2018 so that there is now no cost advantage for Abasaglar. NICE subsequently provided guidance on Abasaglar, accepting its therapeutic equivalence to Lantus and advising that initiation might be undertaken in people naïve to insulin glargine or in those who required dose intensification on Lantus (NICE, 2015b). For those individuals taking Lantus and achieving good glycaemic control and unproblematic hypoglycaemia, a switch to Abasaglar was not recommended.
Semglee
Semglee is another insulin glargine biosimilar licensed for use in the UK. It has been demonstrated to be non-inferior to Lantus (the originator) in terms of HbA1c reduction, with no clinically meaningful differences in hypoglycaemia, safety profile or immunogenicity, in both type 1 diabetes (INSTRIDE-1; Blevins et al, 2018) and type 2 diabetes (INSTRIDE-2; Blevins et al, 2019).
Short-acting insulins
Admelog
Admelog (recently renamed from Insulin lispro Sanofi) is a biosimilar of the rapid-acting human insulin analogue insulin lispro (Humalog). The SORELLA-1 (Garg et al, 2017) and SORELLA-2 (Derwahl et al, 2018) randomised controlled trials indicated similar efficacy and safety (including immunogenicity) of the biosimilar and Humalog in both type 1 and type 2 diabetes (in people using insulin glargine as a basal insulin).
Trurapi
Trurapi is biosimilar insulin aspart that has been shown to be non-inferior in terms of glycaemic control and to have similar safety and immunogenicity profiles as the reference insulin aspart, Novorapid, in both type 1 and type 2 diabetes in the GEMELLI 1 trial (Garg et al, 2020).
Kirsty
Kirsty (previously Kixelle) is a second biosimilar insulin aspart that has recently received EU approval for marketing (EMA, 2020; 2021), although it is not yet available in the UK.
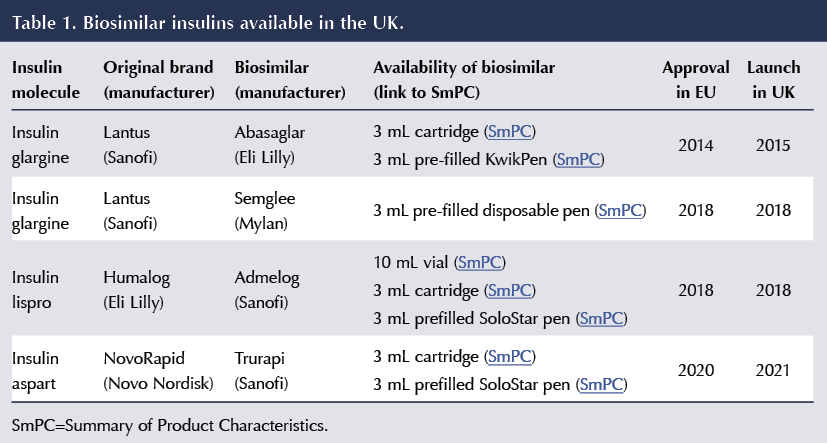
A summary of the biosimilar insulins currently available in the UK, and with their various methods of delivery according to the Electronic Medicines Compendium, is shown in Table 1.
Biosimilar insulin costs
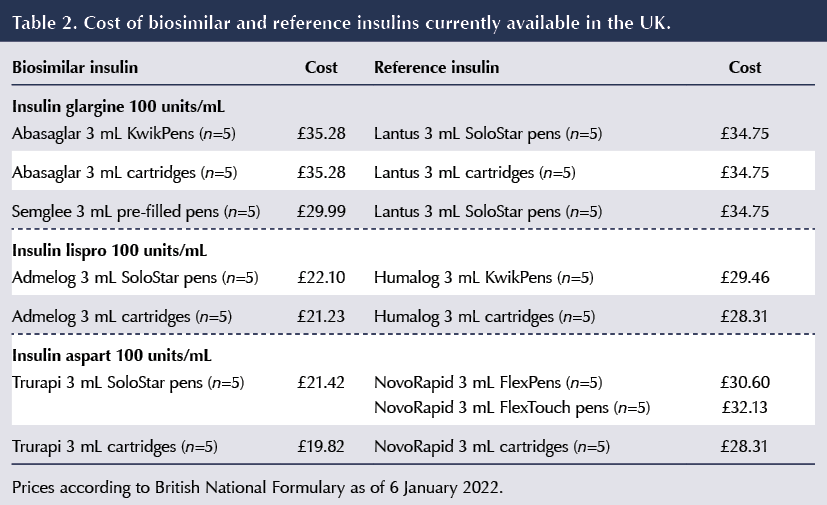
Current prices of biosimilars (with comparison to the reference insulins) is shown in Table 2.
Uptake of biosimilar insulins
The uptake of biosimilar insulins in the UK has been limited (Chaplin, 2021). A UK survey in 2016, following the introduction of Abasaglar, revealed that diabetologists had greater concerns over efficacy and safety of biosimilar products than other disciplines (gastroenterology, rheumatology, dermatology). Only 9% of prescriptions for insulin glargine were for Abasaglar (as opposed to Lantus), a significantly lower proportion than use of biosimilar infliximab in other specialities (e.g. 62% of prescriptions in gastroenterology; Chapman et al, 2017).
A later study found that use of insulin glargine biosimilars had produced savings of £900000 between October 2015 and December 2018, whereas a complete switch would have allowed a saving of £25.6 million in the same period (Agirrezabal et al, 2020). The proportion of insulin glargine prescribed as biosimilars varied widely between Clinical Commissioning Groups (CCGs), ranging from 0% to 53%, which (prior to the reduction in price of Lantus) could be viewed as a missed opportunity to save NHS funds.
NHS England has been strongly in favour of introducing biosimilar medicines, including insulins, because of the potential cost savings, and it has encouraged CCGs to take a lead on promoting their use and supporting clinicians, prescribers and patients in this objective (NHS England, 2017).
Switching to a biosimilar insulin
The decision to switch an insulin to its biosimilar version should not be considered a routine procedure; it must be undertaken by a clinician with a special interest in diabetes, experienced and competent in prescribing insulins, in conjunction with the individual concerned, and never substituted at the point of dispensing (Jayagopal et al, 2018). This advice contrasts with the generic substitution of a simple non-biological molecule, which may be instigated by the pharmacist. Thus, prescribing by brand name is important to avoid inadvertent substitution in the dispensing process (NICE, 2015a). Reassuringly, a systematic review did not find any evidence that switching from a reference biological medicine to a biosimilar led to any major efficacy, safety or immunogenicity issues (Barbier et al, 2020).
For the individual achieving satisfactory glycaemic control without problematic hypoglycaemia, the decision to switch to a biosimilar needs to be weighed up carefully. A detailed guideline and algorithm (Figure 3) for managing the introduction of biosimilar insulin glargine in type 2 diabetes has been produced (Down et al, 2019). The argument here is that, given the equivalent efficacy and safety of the biosimilar insulin, switching could reduce drug budgets and is appropriate for suitable individuals. This approach is supported by the NHS commissioning framework for biosimilars (NHS England, 2017). However, a more conservative approach has been adopted by the Association of British Clinical Diabetologists (Jayagopal et al, 2018) and Diabetes UK (2019), which advise against switching in individuals with stable glycaemic control.

For those individuals with poor glycaemic control, a switch to a biosimilar is reasonable, although the underlying reasons for this should be tackled (Down et al, 2019). It may be a better option to switch to an insulin with different properties to more specifically address the suboptimal glycaemic control.
Initiating a biosimilar insulin
A different scenario is when there has been no previous use of the reference insulin (for example, a newly diagnosed individual with type 1 diabetes or an individual with type 2 diabetes naïve to this class of insulin). In this situation a biosimilar insulin could be considered as first choice. Such a policy may follow the recommendations of the Area Prescribing Committee; however, it must be emphasised that, ultimately, the choice of a biosimilar insulin should be made on an individual basis (Diabetes UK, 2019).
The guideline by Down et al (2019) covers in detail the areas that need to be discussed when an individual commences a biosimilar insulin.
Safety aspects
If an individual switches to a biosimilar insulin, it is essential that they are fully informed and receive appropriate education (Diabetes UK, 2019; Down et al, 2019). The biosimilar insulin may have a different delivery device than the existing insulin (whether based on cartridges or reusable pens), and this needs to be demonstrated to the person. Storage conditions and shelf life may differ from the original insulin molecule according to the Summary of Product Characteristics (Jayagopal et al, 2018).
An individual who has switched to a biosimilar insulin should be advised to monitor their glucose readings more closely to identify any variability in glucose profile compared to the reference insulin (Jayagopal et al, 2018). If the person was previously achieving optimal glycaemic control, it is prudent to start the biosimilar at a 10% lower dose than the originator insulin and then titrate back up to the original dose as necessary. Where there have been persistently low glucose levels or below-target HbA1c, it is appropriate to reduce the starting dose of the biosimilar by 20%. If glucose levels have been running high or HbA1c is above target, the biosimilar should be initiated at the same dose as the originator insulin and uptitrated subsequently as necessary (Down et al, 2019).
Individuals commenced on a biosimilar insulin should be advised to report any possible side effects. The Medicines and Healthcare products Regulatory Agency (MHRA, 2021) has instructed that biosimilar medicines be accorded a black triangle status in their early years of use, indicating that they are under greater scrutiny for safety, with any possible adverse events being reported. If significant, the healthcare professional should report the issue to the MHRA using the Yellow Card system.
Conclusions
Over the last 6 years, biosimilar insulins have become available in the UK; however, uptake has been limited. It is essential that healthcare professionals engaged in the care of people with diabetes, and specifically those with responsibilities regarding the use of insulin, understand the issues around safe and appropriate use of biosimilar insulins and keep abreast of developments. From a scientific point of view, once the biosimilar has demonstrated bioequivalence to the reference insulin, it can be viewed as a reasonable alternative option. The need for cost-saving in the NHS will be the driver of increasing use of biosimilar insulins.
Key points
● Biological medicines are synthesised within living cells. They are large molecules with complex three-dimensional structures.
● A biosimilar medicine is a copy of an existing biological medicine that has no clinically meaningful difference to the original medicine in terms of quality, efficacy, safety or immunogenicity.
● The principal advantage offered by a biosimilar medicine is reduced cost.
● Individuals should only be switched to a biosimilar insulin after discussion with a clinician with expertise in the field. Glucose levels should be carefully monitored after switching.
● Where there has been no previous use of the reference insulin, use of the biosimilar may be considered as a first-line option according to local policies.
● Brand substitution at the point of dispensing is not an acceptable procedure for biosimilar insulins. Thus, prescribing by brand (trade) name is important to avoid the possibility of substitution during the dispensing process.
● Adverse effects that may be attributable to a biosimilar insulin should be reported to the MHRA using the Yellow Card system.




Study provides new clues to why this condition is more aggressive in young children.
14 Nov 2025