The use of glucocorticoid steroids in intercurrent illness is associated with worsening hyperglycaemia both in patients with a known diagnosis of diabetes and in those without the condition. Multiple observational and retrospective data sets suggest an incidence of steroid-induced diabetes of 18–52% in patients commenced on glucocorticoid therapy (Donihi et al, 2006; Liu et al, 2014). Common clinical areas prescribing high-dose steroids are respiratory medicine, oncology, gastroenterology and rheumatology. Despite the high incidence of steroid-induced diabetes, clinical outcomes of this complication are not well reported. Glucocorticoids are an essential part of the management of inflammatory disease processes, allergic reactions, immunosuppression and side effects of malignancies. However, they have profound effects on glucose metabolism, particularly on postprandial hyperglycaemia.
Pathophysiology of steroid-induced hyperglycaemia/diabetes
Synthetic glucocorticoids mimic the effect of the endogenous steroids, nuclear hormones that cross the cell membrane to bind to specific receptors in the cytoplasm of target cells to form glucocorticoid–receptor complexes. Steroid treament modulates carbohydrate metabolism via complex mechanisms, including effects on beta-cell function as well as inducing insulin resistance via effects on insulin receptors in liver, muscle and adipose tissue (Hwang and Weiss, 2014).
In pharmacological doses, glucocorticoids increase insulin resistance. The use of steroids in people with pre-existing diabetes will most often exacerbate their diabetes control and raise their blood glucose levels, precipitating the need for temporary additional treatment. This is known as steroid-induced hyperglycaemia. However, this rise in glucose can also occur in a person who ordinarily does not have diabetes. This is known as steroid-induced diabetes and may resolve once the steroids have been discontinued. The hyperglycaemic effects of steroids are usually transient and dose-related. Steroid-induced diabetes most likely precipitates or “unmasks” diabetes in at-risk individuals (Clement et al, 2004). Glucose levels in most individuals can be predicted to rise approximately 4–8 hours following the administration of oral steroids and sooner following the administration of intravenous steroids.
Clinical implications
Hyperglycaemia in the inpatient setting is associated with a significant increase in hospital-acquired infection, longer hospital stay, higher incidence of intensive care admission, reduced functional independence on discharge and overall increased patient mortality (Suh and Park, 2017). While short courses of steroids resulting in minimal periods of hyperglycaemia may not warrant intervention, higher-dose steroids for longer periods may result in significant symptomatic hyperglycaemia (symptoms including fatigue, polyuria and polydipsia), with the potential for acute complications related to hyperglycaemia.
Hyperglycaemic control in such circumstances will ameliorate symptoms, reduce the risk of acute complications and lessen the risk of infections and other complications associated with hyperglycaemia (Tamez-Pérez et al, 2015). Importantly, hyperosmolar hyperglycaemic state (HHS) and diabetic ketoacidosis (DKA) in the context of steroid use are avoidable complications with significant morbidity and mortality. Capillary blood glucose monitoring is paramount to guiding appropriate therapeutic interventions.
Guidelines for treatment
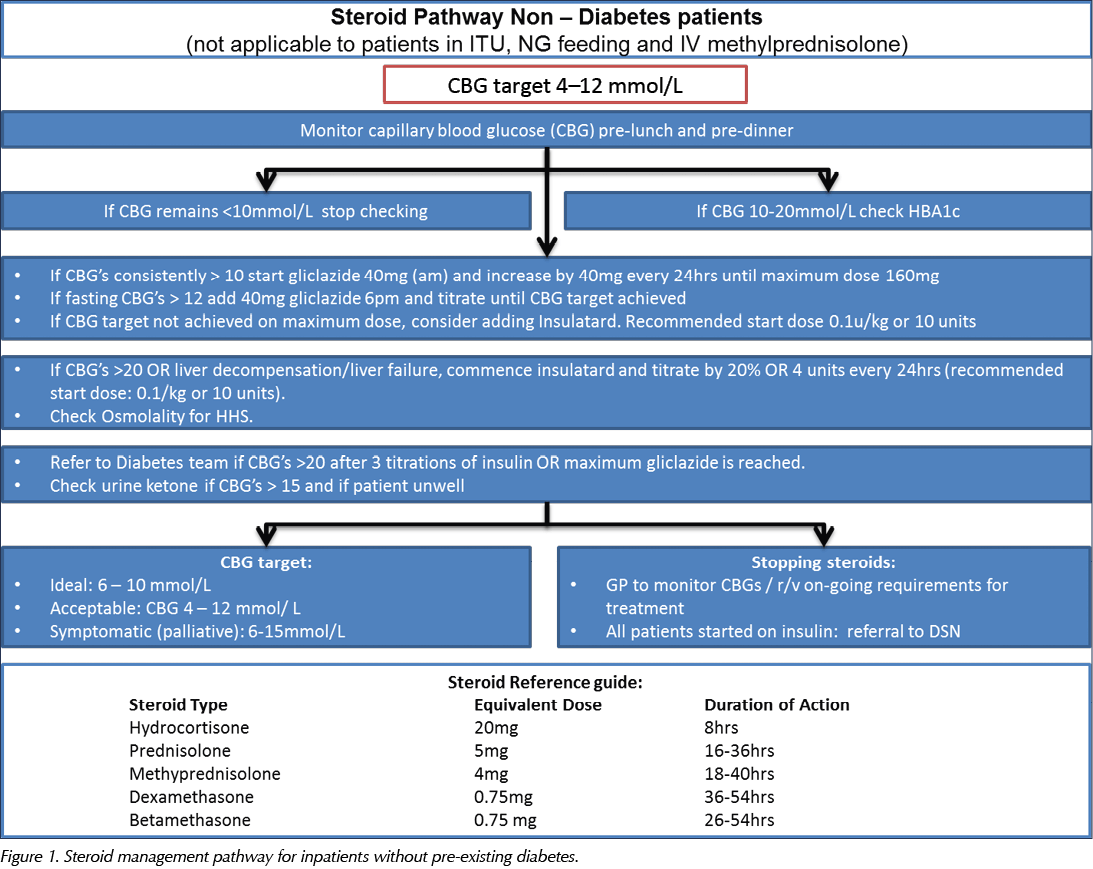
In line with Joint British Diabetes Societies for Inpatient Care (JBDS) guidelines, the recommended target level for blood glucose in hospital inpatients is 6–10 mmol/L, accepting a range of 4–12 mmol/L (JBDS, 2014). However, certain patient groups do not require such tight control, such as people with delirium, dementia or frailty, those at risk of falling, and those with variable appetite and dietary intake. Thus, individualised targets and an appropriate care plan should be documented when hyperglycaemia is first identified, being mindful of the symptoms associated with uncontrolled hyperglycaemia. Patients will need to continue monitoring their capillary blood glucose (CBG) if they are discharged on steroids. This is essential, as diabetes treatment will need to be adjusted as steroids are tapered or increased. The discharge plan needs to include strategies to intercept downward or upward trends in glycaemic control. People with in-hospital hyperglycaemia and previously undiagnosed diabetes should be referred to the diabetes team for follow-up and evaluation of diabetes status, with initiation of therapeutic measures if appropriate.
There is a lack of evidence to guide the management of steroid-induced hyperglycaemia, and much of the guidance given by the JBDS is a consensus based on best practice collated from around the UK.
Study aims and objectives
The objective of this study was to evaluate the incidence and management of steroid-induced hyperglycaemia on medical wards in Royal Free Hospital, London. Early recognition and treatment of steroid-induced hyperglycaemia is essential, as major metabolic decompensation (in the form of HHS) and coma can ensue. In addition, the study aimed to establish the level of awareness of and adherence to a new steroid pathway that was introduced as a pilot in selected medical wards.
Steroid pathway
The objectives of the steroid pathway are to:
- Promote awareness of how steroids affect glycaemic control.
- Promote awareness of appropriate glucose testing whilst patients are on steroids.
- Ensure that implementation of treatment is timely.
- Create an understanding of suitable treatment options for people with and without diabetes.
- To maintain stable glycaemic control during steroid therapy and avoid hypoglycaemia and HHS.
- Ensure appropriate follow-up on discharge for patients who have required additional or introductory treatment.
The pathway is designed to provide safe, practical guidance on the screening, diagnosis and management of steroid-induced hyperglycaemia and diabetes. It aims to guide medical staff in appropriate blood glucose monitoring and subsequent decisions regarding the need for additional or introductory treatment and follow-up. The pathway comprises algorithms for treating people without known diabetes and those with pre-existing diabetes, which are detailed in Figure 1 and Figure 2, respectively.
Methods
Three medical wards were selected for the purpose of audit: the respiratory ward, the hepatology ward and the oncology ward. These were selected as their patients generally have acute medical conditions which are more likely to require steroid treatment. The pilot steroid pathway was introduced in these wards in May 2018. The junior doctors on each ward were given an overview of the pathway and copies of it were displayed on the wall in the junior doctors’ office on each ward for reference.
A retrospective review of all medical admissions to three medical wards was performed over a 3-month period from June to August 2018 to evaluate the identification and management of steroid-induced hyperglycaemia. Patients who had been commenced on steroid treatment were identified. Subsequently, to evaluate the awareness of and adherence to the steroid pathway, the patient notes, drug charts and CBG charts of these individuals were reviewed for the following data:
- Age.
- Gender.
- Diagnosis.
- Comorbidities.
- Known history of diabetes.
- HbA1c.
- Length of stay.
- Duration of steroid treatment.
- CBG prior to start of steroid treatment.
- CBG 24 hours after start of steroid treatment.
- CBG 24 hours after first intervention for hyperglycaemia.
- CBG 48 hours after first intervention for hyperglycaemia.
- Was CBG monitored during the entire duration of steroid therapy as per the steroid pathway?
- Were ketone levels checked when CBG was greater than 15 mmol/L?
- When CBG was greater than 20 mmol/L, were patients appropriately referred to the diabetes team as per the steroid pathway?
- Inappropriate referrals made to the diabetes team following introduction of the steroid pathway.
- Was an appropriate diabetes plan made on discharge?
The results were tabulated into an Excel spreadsheet and were analysed to assess for adherence to the steroid pathway and JBDS (2014) guidelines.
Results
A total of 35 people were commenced on steroids in these three wards over the 3-month audit period. Of these, nine had pre-existing diabetes (seven with type 2 diabetes and two with known steroid-induced diabetes).
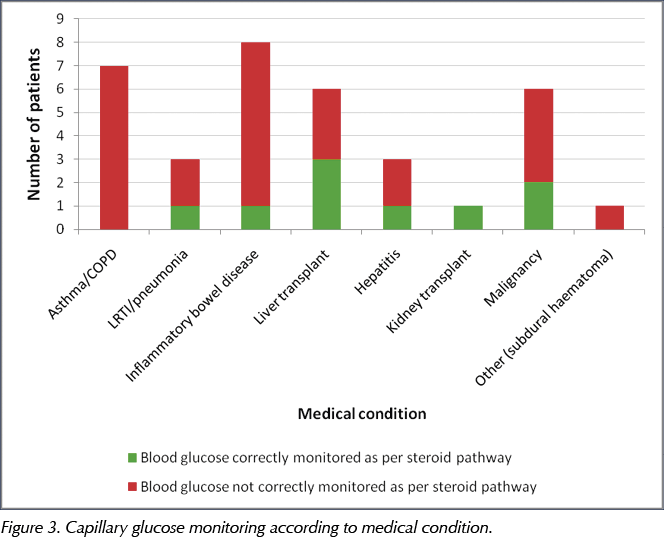
Only 14 (40%) of the 35 patients commenced on steroids had their CBG monitored in the 24-hour period after the steroid had been started. Of the nine patients with a pre-existing diagnosis of diabetes (type 2 or steroid-induced diabetes), almost all (8/9; 89%) had their CBG checked in the first 24 hours after starting on steroids. Only one of the 26 patients without a diagnosis of diabetes had their CBG correctly monitored as per the pathway for the duration that they were on steroids. By medical condition, the proportion of patients who had their CBG monitored correctly according to the pathway is shown in Figure 3.
Of the 14 patients commenced on steroids who had their CBG checked post-introduction of steroids, ten (71.4%) developed steroid-induced hyperglycaemia. Of these, nine had a pre-existing diagnosis of diabetes. Of those patients who developed steroid-induced hyperglycaemia and required an intervention to manage it, all (10/10) had their CBG monitored appropriately at 24 and 48 hours after the commencement of steroids.
The steroid pathway states that ketones should be checked if CBG is greater than 15 mmol/L, and it also informs doctors of when the management plan should be escalated to the diabetes team. Only one of the 13 patients (7.7%) who developed hyperglycaemia greater than 15 mmol/L had their ketones checked correctly as per the pathway. However, 60% (6/10) of patients who developed hyperglycaemia greater than 20 mmol/L were appropriately referred to the diabetes team.
After the introduction of the steroid pathway in the three medical wards, 30% (3/10) of referrals received by the diabetes team were inappropriate, in that these patients could have been appropriately managed with use of the steroid pathway.
All patients had appropriate diabetes plans in place on discharge.
Are we meeting JBDS standards?
The results in Table 1 show that, with the exception of discharge planning, the three wards involved in this audit have not met the JBDS (2014) standards.
Discussion
Nationally, approximately 18% of hospital beds are occupied by a person with diabetes (NHS Digital, 2018). In this study, 26% of patients who were commenced on steroids had pre-existing diabetes, and so were already at significant risk of developing steroid-induced hyperglycaemia.
There appears to be poor uptake of the identification of steroid-induced hyperglycaemia and steroid-induced diabetes, whereby only 40% of patients started on steroids had their CBG checked in the first 24-hour period after the steroid had been commenced (compared with JBDS audit standards of 90%). Of these 14 patients, eight had known diabetes. It may be considered that the limited compliance with CBG monitoring may have been achieved primarily through the routine monitoring of CBG in people with diabetes (which may have even taken place regardless of whether the patient was on steroids or not). Nonetheless, it can be concluded that patients with pre-existing diabetes are more likely to have their CBG monitored regularly in hospital, and thus have any steroid-induced hyperglycaemia detected, compared to people without diabetes, who may not have their CBG monitored and in whom steroid-induced diabetes may therefore be missed.
Interestingly, 100% of the patients with diabetes had their CBG correctly monitored as per the steroid pathway for the duration of being on steroids. However, only one of the remaining 26 patients without diabetes had their CBG correctly monitored as per the pathway. Again, this demonstrates that people with diabetes had their CBG monitored regularly whilst they were on steroids, in accordance with the steroid pathway. In people without diabetes, however, steroid-induced hyperglycaemia appears to be undervalued in terms of diagnosis and treatment.
In the general medical population, steroid treatment is being administered for respiratory disease in up to 40% of patients (JBDS, 2014). In this audit, 29% of the patients (10/35) had presented with a respiratory condition requiring steroid therapy. Exacerbations of chronic obstructive pulmonary disease (COPD) and asthma are common conditions that are treated with steroids. People with COPD tend to have an increased risk of cardiovascular disease and factors that can predispose to diabetes, including older age, hypertension and obesity. Therefore, when treated with steroids, such individuals can be at increased risk of developing steroid-induced hyperglycaemia and require monitoring to enable early identification of this complication. Unfortunately, of the ten patients presenting with respiratory conditions (of whom six had COPD exacerbations), only one was appropriately monitored for steroid-induced hyperglycaemia and had their CBG checked within the first 24 hours of commencing steroid treatment (Figure 3).
Ten of the 35 patients in this cohort (28.6%) were found to have developed steroid-induced hyperglycaemia; however, only 40% of the cohort had their CBG checked in the first 24 hours after the steroid had been commenced. This suggests that more cases of hyperglycaemia may have been detected if CBG had been appropriately checked in all patients commenced on steroids, and that the actual incidence of steroid-induced hyperglycaemia was actually higher than 28.5%.
Unsurprisingly, of the ten patients who developed steroid-induced hyperglycaemia, nine had pre-existing diabetes. This also means that all the patients with known diabetes developed steroid-induced hyperglycaemia, which illustrates the concept that steroids worsen glycaemic control in people with diabetes. However, it is difficult to draw conclusions regarding the incidence of steroid-induced diabetes in patients without diabetes due to the lack of CBG monitoring. Furthermore, the one patient who was observed to develop steroid-induced diabetes was not appropriately screened for pre-existing diabetes with an HbA1c test.
Good compliance with the steroid pathway was observed with regard to CBG monitoring (at 24 and 48 hours after starting steroids) once interventions had been made to manage steroid-induced hyperglycaemia. Again, as most of these patients had a pre-existing diagnosis of diabetes, it is unclear whether the CBG monitoring was to check for the effect of steroids or purely for the monitoring of the patients’ diabetes.
After the introduction of the steroid pathway in the three medical wards, only 7.7% of patients with hyperglycaemia of more than 15 mmol/L had their blood ketone levels checked as per the pathway. Reviewing the pathway, it appears that this practice is not highlighted clearly. Furthermore, 30% of referrals received by the diabetes team (3/10) were inappropriate, in that these patients could have been appropriately managed by following the steroid pathway. This strongly suggests that the pathway needs to be reviewed and should be reworded and rearranged to make it more user-friendly and clear.
Of the ten patients who developed hyperglycaemia persistently greater than 20 mmol/L, four (40%) were not referred to the diabetes team as advised by the steroid pathway. Again, the pathway needs to be reviewed to ensure that such salient points are highlighted. Failure by the ward doctors to identify such patients and subsequently get specialist advice can increase the patients’ risk of developing DKA and HHS. Unfortunately, low adherence to the steroid pathway guidance is likely to have contributed to the lack of adequate blood glucose control (despite interventions) for patients who developed steroid-induced hyperglycaemia or steroid-induced diabetes.
All patients in this audit had appropriate diabetes plans in place on discharge. This met the JBDS (2014) audit standards.
Conclusions
Steroid-induced hyperglycaemia is common, and potentially harmful, in medical inpatients. However, its diagnosis and treatment are surprisingly undervalued by most healthcare professionals. It is also important to note that acute illness may result in “stress hyperglycaemia” independent of steroid administration (Dungan et al, 2009). Some patients may develop hyperglycaemia at a lower steroid dose, so clinical vigilance is recommended with steroid therapy at any dose, to enable early identification of hyperglycaemia and implementation of appropriate management strategies. Practice variation exists, and some practices may be deemed suboptimal. The knowledge that hyperglycaemia leads to longer hospital stays, delayed wound healing, increased risk of infection and higher mortality rates should encourage healthcare providers to monitor its occurrence and manage it appropriately. This local audit provides a foundation for future quality improvement projects.






Study provides new clues to why this condition is more aggressive in young children.
14 Nov 2025