Diabetic foot ulcers (DFUs) are among the most serious complications of diabetes, affecting an estimated 6.3% of the global diabetic population, or roughly 440 million individuals worldwide (Thanigaimani et al, 2021). They are a leading cause of hospitalisations and lower-limb amputation, and carry a five-year mortality rate as high as 40%, rivalling many cancers (Armstrong et al, 2020).
Despite widespread implementation of the current standard of care (SOC), which includes debridement, offloading, infection control, moisture management, and systemic management of underlying conditions, many DFUs remain unhealed for months, or even years. Recurrence is also a major challenge, with over 50% of ulcers reoccurring within 3 years (Frykberg et al, 2020; Thanigaimani et al, 2021). Even when wounds heal, reoccurrence is common, and many DFUs fail to respond at all to the SOC, particularly in patients with ischemic or complex comorbidities (Yellin et al, 2022).
Method
In a randomized controlled trial to evaluate the efficacy of cyclical Topical Wound Oxygen Therapy (TWO2) in the treatment of chronic DFUs (Frykberg et al, 2020) it was established that using TWO2 therapy produced wounds that were six times more likely to heal in 12 weeks compared with the SOC alone. Additionally, at 12 months, the recurrence rate for wounds treated with TWO2 therapy was six times lower than that for SOC alone.
The treatment phase of the study was 12 weeks. The randomisation visit measurement after debridement served as the index (baseline) measurement. If multiple ulcers were present, the largest area ulcer at the baseline visit was designated the index ulcer. Weekly digital wound images were transmitted electronically and were assessed for area changes and closure confirmation by a single blinded central assessor, using automated CE-marked wound measurement software. Once a wound was initially determined to be closed by the blinded study site investigator, that visit served as the first of two confirmatory visits. Wound closure (complete epithelialisation) was confirmed at the second closure visit two weeks later. Upon completion of the 12-week treatment phase, patients entered the post treatment follow-up period for an additional 38 weeks, whereby they returned for wound closure assessment and quality of life questionnaires. The maximum duration for participation in the study was 54 weeks. During the follow-up phase, patients without healed ulcers received standard care according to their clinician’s recommendation and were asked not to participate in another wound care trial. The primary study end point was the percentage of ulcers in each group achieving 100% healing at 12 weeks. Secondary end points included wound area reduction, 12-month incidence of both recurrence and complete healing, incidence of amputation, Cardiff Wound Impact Schedule (CWIS) assessment, and adverse events.
Statistical analysis
All analyses were performed solely on the intention-to-treat study population using Stata 12 (StataCorp, College Station, TX). Results are reported to one decimal place; P values and SDs have been reported to two significant figures. For the primary end point of ulcers achieving 100% healing at 12 weeks, statistical significance was assessed at the Pocock 2.2% level (P < 0.022). Logistic regression analysis was used to determine the influence of possible confounding variables. Model diagnostics were used to check regression model assumptions and transformations if they did not hold. For this analysis, a backward elimination process was used incorporating the following variables: age, sex, ulcer area, ulcer duration, presence of neuropathy, UTC grade, and HbA1c (%). The same potential confounders were examined within the Cox proportional hazards model. Confounders were included in both models if they changed the odds ratio (OR) or hazard ratio (HR) by more than 10%. The final logistic regression model and longitudinal hazard models included 97.8% confidence intervals (CIs). For all other analyses, statistical significance was assessed at the two-sided 5% level (P < 0.05) with 95% CIs provided as appropriate. The statistician conducting all analyses was blinded to treatment allocation (with groups identified as A and B) until results had been finalised.
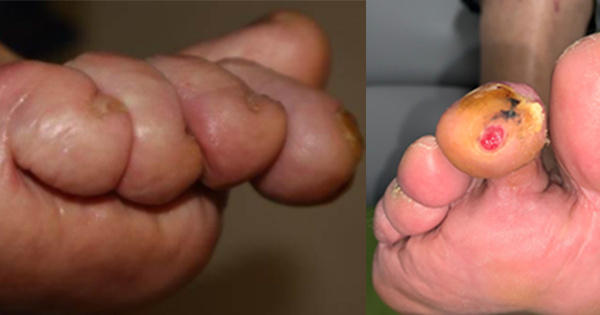
A post hoc analysis of this study revealed that even moderately ischemic wounds can be successfully treated using this technology. According to IWGDF criteria, these wounds are defined by any of the following: ABI ≥ 0.7, TBI < 0.75, monophasic or biphasic Doppler wavess below the knee, TCPO2 < 60, great toe pressure < 60, or skin perfusion pressure < 60 (Figure 1).
The study inclusion criteria were as follows: diabetic patients with non-healing, full-thickness DFUs of UT grade 1 or 2, measuring between 1cm2 and < 20 cm2 post debridement. The duration of the DFU was between 4 weeks and 1 year, and all had received standard care for at least 4 weeks.
Patients received optimal standard care, including sharp debridement, foam dressings, and a below-knee offloading device equivalent to a total contact cast. Patients who then failed a two-week run-in period with the standard care received either a TWO2 therapy device or sham device; both devices looked and operated identically. Moderate ischemia, according to IWGDF criteria, was defined as any of the following: ABI ≥ 0.7, TBI < 0.75, monophasic or biphasic Doppler waves below the knee, TcPO2 < 60, great toe pressure < 60, or skin perfusion pressure < 60.
TWO2 therapy mechanism of action
In chronic DFUs, ischemia and impaired microvascular circulation severely restrict the delivery of oxygen to the wound bed, disrupting the cellular processes essential for healing. TWO2 therapy addresses this barrier by saturating the wound with up to 10 litres of oxygen per minute, creating a high-pressure environment that establishes a steep diffusion gradient across the wound surface. This mechanical force drives oxygen into the hypoxic tissue, even in areas with poor perfusion, thereby restoring oxygen availability at the cellular level.
By delivering cyclical pressurised oxygen directly to the wound site, TWO2 therapy alters the wound healing environment. The introduction of high levels of oxygen under pressure elevates tissue oxygen tension while simultaneously lowering the wound pH. This dual effect reshapes the biochemical landscape of the wound, creating conditions that are far more favourable for repair and regeneration.
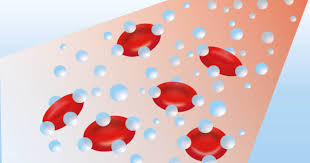
On a cellular level, this oxygen rich environment stimulates the upregulation of several key enzymes that are crucial to the healing cascade (Figure 2). Firstly, NADPH oxidase, one of the body’s primary antimicrobial enzymes, is activated to help control infection and reduce bioburden. In parallel, ATP synthase, the enzyme responsible for generating energy for all other enzymatic reactions, is enhanced, providing fibroblasts and other repair cells with the energy required for sustained activity. Additionally, enzymes such as prolyol hydroxylase and lysly hydroxylase, both of which are essential in fibroblast function, are upregulated. Their activation improves the cross-linking of collagen fibers, strengthening the structural matrix of the healing tissue. Finally, nitric oxide synthase is stimulated, which promotes angiogenesis and capillary bed formation, thereby improving blood flow and nutrient delivery to the site of injury. Together, these enzymatic responses cause the wound to begin healing and initiate the critical process of granulation tissue formation.
Results
A total of 18 patients were included in each of the two groups: the study group and sham group. At 12 weeks, seven out of 18 patients (39%) in the TWO2 therapy group healed completely, compared with zero of 18 patients (0%) in the sham group (P < 0.0076).
Discussion
In addition to oxygenation, TWO2 therapy provides two adjunctive mechanisms that further optimize the wound healing environment:
- non-contact cyclical compression; and
- humidification.
The non-contact cyclical compression mobilises both blood and lymphatic fluids, reducing oedema and periwound oedema. This oedema reduction lowers hydrostatic pressure in the tissues, allowing for improved arterial inflow and enhanced microvascular prefusion. The resulting improvement in oxygen diffusion facilitates neovascularisation, supports granulation tissue formation and contributes to the resolution of chronic inflammation. Meanwhile, humidification allows for better oxygen diffusion and contributes to an ideal wound healing environment.
Together, these modalities create a physiologically balanced wound environment conducive to faster and more durable healing.
Conclusion
This randomised study demonstrated that moderately ischemic diabetic foot ulcers had significantly higher healing rates using TWO2 therapy than with the sham treatment. TWO2 therapy provides a multimodal approach to achieve higher healing rates by increasing oxygenation, providing non-contact compression and humidification. Uniquely, the therapy has an additional benefit in that it can be administered by the patient at home without the expense and difficulties of daily travel to a specialised center. This robust double-blinded, sham-controlled trial provides evidence to support the use of this adjunctive cyclical pressurised topical oxygen therapy for chronic DFUs.