Foot infections are now among the most common, and potentially devastating, complications of diabetes. Most diabetic foot infections (DFIs) start in a foot wound, usually one that results from the consequences of peripheral neuropathy (sensory, motor, autonomic), often with peripheral vascular disease in the background.
Infection occurs when organisms, usually the aerobic Gram-positive cocci colonising the surrounding skin, proliferate and cause a host response. This is manifest as signs and symptoms of inflammation, followed by tissue destruction. Unchecked, infection can progress contiguously to involve more of the superficial, and often the deeper, soft tissues. Ultimately, underlying bone becomes infected in about 20% of DFIs (Lipsky and Berendt, 2008).
Diabetic foot infection guidelines have been published by an expert panel selected by the Infectious Diseases Society of America (IDSA; Lipsky et al, 2012a) and the International Working Group on the Diabetic Foot (Lipsky et al, 2012b), and represent updates of guidelines published by each of these groups in 2004. The format of the new IDSA DFI guidelines largely consists of posing questions, answering them, and providing and grading the evidence used for the answers. The 10 questions can be summarised as follows:
1. In which patients with diabetes and a foot wound should I suspect infection, and how should I classify foot wounds?
Consider the possibility of infection in any foot wound in a person with diabetes; those with neuropathy, a previous foot wound or amputation, peripheral arterial disease, or renal insufficiency are at increased risk. Clinicians should be attuned to the signs and symptoms of life- or limb-threatening DFI, which are summarised in Box 1.
Classify wounds using a validated system; for infection, this includes the IDSA definitions of uninfected and mild, moderate, and severe infection (Box 2; Table 1). Using a validated wound scoring system may be helpful to follow progress during treatment (Lipsky et al, 2009).
2. How should I assess the patient with diabetes presenting with a foot infection?
Evaluate the patient at three levels: the whole patient, the affected limb, and the wound. Diagnose infection by the presence of classic signs of local infection (Box 2), and occasionally by the presence of various secondary findings (e.g. undermining, poor-quality granulation tissue) in the wound.
Assess the patient for evidence of clinically significant peripheral ischemia, which may require revascularisation. Assess the need to debride any necrotic tissue or surrounding callus.
3. When should I request a consultation for a patient with a DFI, and from whom?
Attempt to provide a well-coordinated approach to management for both outpatients and inpatients with DFIs, including the involvement of appropriate specialists (e.g. podiatrists, orthopaedic or vascular surgeons, infectious diseases specialist) when needed. This is best accomplished within the setting of a multidisciplinary foot team, but otherwise should be coordinated by a designated clinician. Seek expertise in providing optimal pressure offloading of the wound, if needed.
4. Which patients with a DFI should I hospitalise, and what criteria should they meet before being discharged?
Hospitalisation is by far the most expensive aspect of managing a DFI and is needed only in specific situations, including:
- Those with a severe infection
- Those requiring inpatient diagnostic or therapeutic procedures.
- Those with complex wound care requirements
- Those with psycho–social issues that preclude outpatient treatment.
Prior to discharge, the patient should have had any urgent procedures performed, be clinically stable, be able to manage as an outpatient, and have a well-defined treatment follow-up plan.
5. When and how should I send specimen/s for microbiological culture from a patient with a diabetic foot wound?
It is unnecessary to culture clinically uninfected wounds, but clinicians should obtain appropriate specimens from most wounds with evidence of infection. These should preferably be deep-tissue specimens, obtained by biopsy or curettage after wound cleansing and debridement, or aspirates of purulent secretions. Wound surface swab specimens are likely to yield colonising organisms and miss true pathogens and should be avoided.
6. How should I initially select, and when should I modify, an antibiotic regimen
for a DFI?
While all foot lesions require appropriate wound care, only clinically infected wounds need antibiotic treatment. An empiric antibiotic regimen, based on the severity of the infection and the likely aetiological agent/s, should be initially selected (Table 2; Table 3).
For mild to moderate infections in patients who have not recently received antibiotic treatment, targeting just aerobic Gram-positive cocci (especially Staphylococcus aureus) is usually sufficient. For severe infections, initiate broad-spectrum empiric therapy, pending the results of culture and sensitivity testing. Empiric therapy directed at Pseudomonas aeruginosa is usually unnecessary, except in those patients with risk factors for true infection with this organism. Consider providing empiric therapy directed against methicillin-resistant S. aureus (MRSA) in patients with a history of MRSA infection, when the local prevalence of MRSA colonisation or infection is high, or if the infection is clinically severe.
Routes and length of antibiotic therapy are summarised in Table 4. Base the route of therapy largely on infection severity, with parenteral therapy for all severe, and some moderate, DFIs, at least initially, and switch to oral agents when the patient is systemically well and culture results are available. Use highly bioavailable oral antibiotics in most mild, and in many moderate, infections. Consider topical antimicrobial therapy for selected mild superficial infections.
Base definitive antibiotic therapy on the results of culture and sensitivity testing of an appropriately obtained wound specimen, as well as the patient’s clinical response to the empiric regimen. Continue antibiotic therapy until, but not beyond, resolution of the infection, but not through complete healing of the wound; for soft tissue infections this will usually be approximately 1–3 weeks.
7. When should I consider imaging studies to evaluate a DFI, and which should I select?
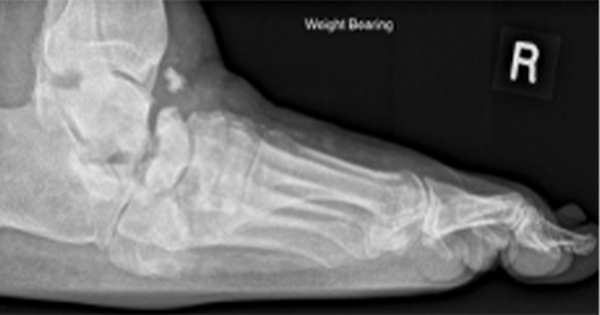
Order plain radiographs for almost all patients presenting with a new DFI to look for bony abnormalities, soft tissue gas, and radio-opaque foreign bodies. For patients who require more sensitive or specific imaging, particularly when soft tissue abscess or osteomyelitis is suspected, magnetic resonance imaging (MRI) is the study of choice. If MRI is unavailable or contraindicated, consider the combination of a radionuclide bone scan and a labelled white blood cell scan.
8. How should I diagnose and treat osteomyelitis of the foot in a patient with diabetes?
Consider the possibility of osteomyelitis in the presence of any infected, deep, or large foot ulcer, especially one that is chronic or overlies a bony prominence. A properly performed and interpreted probe-to-bone test in a DFI with an open wound can help to diagnose diabetic foot osteomyelitis (high likelihood) or exclude it (low likelihood).
Plain X-rays have relatively low sensitivity and specificity for confirming or excluding osteomyelitis, but serial X-rays over a period of weeks may be useful. MRI is the best of the advanced diagnostic imaging studies for osteomyelitis, but is usually needed only when diagnostic uncertainty remains after clinical and plain X-ray evaluations.
The definitive diagnosis of osteomyelitis rests on bone culture, combined with histopathology. Bone biopsy is most appropriate when there is diagnostic uncertainty, inadequate culture information, or failure of response to an empiric treatment.
Consider using either primarily surgical or primarily medical strategies for treating diabetic foot osteomyelitis in properly selected patients. The duration of antibiotic therapy can be short (2–5 days) when surgical resection leaves no remaining infected tissue, but should be more prolonged (≥4 weeks) when there is persistent infected or necrotic bone.
9. In which patients with a DFI should I consider surgical intervention and what procedures may be appropriate?
Consider urgent surgical intervention for DFIs accompanied by gas in the deeper tissues, an abscess, or evidence of necrotizing fasciitis, and less urgent surgery for wounds with substantial nonviable tissue, or extensive bone or joint involvement in the infection. The surgeon should have experience managing patients with DFIs and knowledge about foot anatomy.
10. What type of wound care techniques and dressings should I use for a patient with a diabetic foot wound?
Key aspects of wound care include adequate cleansing, debridement of callus and necrotic tissue, selecting an appropriate dressing that will allow for moist wound healing while controlling excess exudate, and pressure offloading. When an infected wound is unresponsive to treatment, consider whether the problem is a failure of the infection to respond (due to untreated ischemia, inadequate drainage or debridement, pathogens that are resistant to the prescribed therapy, or lack patient adherence to the antibiotic regimen) or failure of the wound to heal (because of lack of adherence to the dressing regimen or offloading device, misdiagnosis of the cause of the wound, or inadequate blood flow).
Among adjunctive measures, hyperbaric oxygen therapy may help heal wounds more quickly, but has not been shown to improve infection outcomes, and granulocyte-colony stimulating factors may reduce the need for various surgical interventions, but do not appear to improve other infectious outcomes.
Conclusions
The IDSA expert panel recommend several areas that would be most useful to investigate to improve the management of DFIs in the future. They include those related to implementation of available guidelines (e.g. deploying multidisciplinary teams, developing audit systems for both the process and outcomes of treatment, and encouraging clinicians and healthcare providers to assess and improve their outcomes) and those related to regulatory changes (e.g. developing guidance for studies of new agents for treating DFIs that will lead to marketing approval, including for osteomyelitis). Helpful future studies would elucidate the role of biofilm in DFIs and how best to manage it, and the potential usefulness of molecular microbiological techniques in DFI management.
In the past decade there has been an enormous increase in studies examining the epidemiology, pathophysiology, treatment, and outcome of DFIs. The evidence base for managing DFIs is now robust enough to allow good outcomes – especially the avoidance of major amputations – in the majority of patients. Efforts now must focus on the implementation of what we know works, while continuing to find better ways to manage this complex and potentially devastating problem.