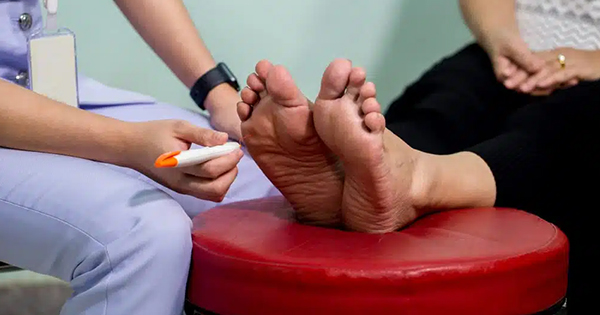
Slough is a complex, generally fibrinous, mass that consists variously of fibrin, deoxyribonucleo-protein, leucocytes, bacteria, proteinaceous material, and serous exudate (Thomas, 1997; Black et al, 2010). Slough is commonly found on diabetic foot wounds (Figure 1) and may or may not be firmly attached to surrounding tissue (i.e. both dry and moist presentations). The longer it is present in a wound, slough may become thicker and more difficult to remove (Black et al, 2010).
Slough acts variously as a retardant for healthy wound bed granulation, a barrier to viewing the depth and extent of a wound, a reservoir for pathogenic organisms, and a source of malodour. The clinician must consider the presence of slough to be clinically significant, and seek to remove slough to prepare the wound for healing.
Slough and infection
The generation, appearance, and regeneration of slough at the wound site is considered to be linked to bacterial activity (Harding and Enoch, 2003). The composition of slough is such that it is a medium for pathogenic microorganisms, with the result that it may act as a reservoir for infection that may threaten the patient’s limb, or as source of malodour that is distressing to the patient.
Slough ranges in colour, with white slough being suggestive of low bacterial counts, to yellow or green slough suggesting higher bacterial counts. Slough may present with a brown tinge, indicating haemoglobin is present. Slough should not be confused with normal anatomical tissues (e.g. tendons, ligaments), although the yellow colour of both has led to the mistaking of one for the other (Black et al, 2010).
Recurrent slough may be associated with biofilms – complex structures of microbial-associated cells embedded in self-produced extracellular matrix, which attach irreversibly to a biological or nonbiological surface and make the indwelling bacteria resistant to traditional therapies (Davis et al, 2006). The rapid reformation of slough following a successful episode of cleansing or debridement is suggestive of the presence of biofilm.
Slough and the diabetic foot
The lifetime risk of a person with diabetes developing a foot ulcer is reported to be as high as 25% (Boulton et al, 2008) and these wounds require expert treatment. Cleansing and debridement represent important elements of the management of a range of wounds types (Strohal et al, 2013), especially the vulnerable diabetic foot (Levin, 2002). It is vital to carry out a full investigation of the patient and wound prior to undertaking these activities, as this will assist in identifying the methods that are most appropriate for the wound and the patient. Full discussions of the assessment of the diabetic foot, and the range and selection of debridement methods, can be found in McInnes’ (2001) Guide to the Assessment and Management of Diabetic Foot Wounds, and Effective Debridement in a Changing NHS (Wounds UK, 2013), respectively. Here, the authors concentrate specifically on the removal of slough from diabetic foot wounds.
Managing slough
The various negative roles that slough can play in diabetic foot wounds mean that cleansing or debridement to remove these debris must be undertaken.
Sharp debridement
While surgical or sharp debridement are rapid methods for the remove of wound debris, they not always practicable or most appropriate (Thomas, 1997) – with both these techniques being the preserve of highly-trained and -skilled clinicians (traditionally physicians, specialist podiatrists, and podiatric surgeons), and under no circumstances to be attempted by the novice.
A range of other techniques for the removal of wound debris – spanning cleansing and debridement – are available to clinicians with a range of backgrounds. Wound irrigation, the use of cleansing solutions or a cleansing pad (e.g. Debrisoft®; Activa Healthcare), or the use of dressings – such as hydrogel sheets, honey or iodine cadexomers – can be used to remove slough by clinicians with minimal training.
Wound cleansing
Low levels of loose wound slough maybe removed simply by thorough, traditional cleansing. This can be achieved using tap water, sterile water, or saline; debates about the appropriateness of each of these cleansing solutions for the diabetic foot remain, and it is important that the clinician assess the risk of infection and cost–benefits associated with each (Watret and McClean, 2009).
Soaks may be used to remove wound debris (e.g. sodium hypochlorite, hydrogen peroxide), but are in recent times considered to adversely effect the healing process, especially in the delicate and vulnerable tissues of the diabetic foot.
Other proprietary cleansing solutions are available, the most widely used of these being Prontosan® Wound Irrigation Solution (B. Braun; also available as a gel formulation), the key ingredients of which are polyhexamethylene biguanide (PHMB), an antimicrobial agent, and betaine, a surfactant. This product is primarily for the decontamination of wounds that are at risk of infection by aiding the removal of bacteria and debris, and disrupting biofilm.
Mechanical debridement
Mechanical debridement is a nonselective, physical method of removing nonviable tissue and debris from a wound using mechanical force. These modalities can include “wet-to-dry” (never used in the UK), pressurised irrigation, ultrasound, and through topical negative pressure. Though generally easy to perform and rapid, traditional mechanical debridement methods can damage healthy granulation tissue, both in the wound bed and at the margins of the wound due to their nonselective nature (Enoch and Harding, 2003).
Another mechanical product is Debrisoft. This unique product is a single-use debridement pad that consists of monofilament polyester fibres with a reverse side of polyacrylate. The fibres are cut with angled tips designed to remove devitalised tissue, debris, and hyperkeratotic skin caused by chronic and acute wounds (Haycocks and Chadwick, 2012).
Debrisoft is designed to be moistened (with tap water, saline, or an antimicrobial solution, as appropriate) and applied with light pressure to the wound in circular motions for 2–3 minutes to loosen and collect the devitalised tissues, and then discarded. This is a simple method that can be used by experienced and inexperienced clinicians alike.
Larval debridement
Larval debridement is the medical use of live fly larvae for treating necrosis or hard-to-remove slough from wounds. It is an ancient method, used historically by military surgeons (Steenvoorde et al, 2007) and the ancient Chinese (Chan et al, 2007).
This therapy use increased in the 1930s and the effectiveness of maggots in cleansing wounds is now accepted practice and available on prescription. The maggots are produced in laboratories and are disinfected to ensure they are safe in human tissue.
Three proteolytic enzymes have been identified in maggot excretions/secretions (Chambers et al, 2003) which effectively degrade extracellular matrix components.
One study (Sherman, 2003) noted that after 5 weeks of therapy, conventionally treated wounds remained necrotic over 33% of their surface, whereas, after only 4 weeks, maggot-treated wounds were completely debrided. Marineau et al (2011) identified that maggots are able to debride diabetic wounds and stimulate wound healing whereas Paul et al (2009) found no significant difference in outcome between conventional debridement and maggot therapy.
In a recent meta-analysis (Tian et al, 2013), four studies that compared maggot debridement with standard therapy on a total of 356 participants with diabetic foot ulceration were investigated. The results suggested that the maggot-treated group was significantly superior to the control group in the percentage of diabetic foot ulcers to achieve full healing (P=0.03), amputation rate (P=0.02), time to healing (P=0.0004) and number of antibiotic-free days (P=0.001). The authors concluded that, while maggot therapy is undoubtable effective in treatment of diabetic foot ulcers, larger studies are needed before it is routinely recommend for treatment (Tian et al, 2013).
Maggot treatment is deployed using either “free range” maggots (Larvae300; BioMonde) or those contained within a dressing. Some clinicians find it difficult to apply the free range maggots, but use of this modality means that the maggots are free to fully access the devitalised tissues and is especially useful when the depth and extent of the wound is not known. For convenience, there are also maggots contained within a finely woven net pouch, and the pouch can be placed within the wound (BioBag; BioMonde).
Dressings for the management of slough
Autolytic debridement is the natural process by which enzymes soften and liquefy devitalised tissues – a key component of the removal of slough by the body’s own systems. The use of dressings is a common and simple method of supporting autolytic debridement, and a range of suitable dressings are available for this purpose.
Hydrogel sheets
Hydrogel sheets have been successfully used in promoting autolytic debridement of wounds, although caution should be used in patients prone to maceration as these products can increase exudate in the periwound area. Examples of hydrogel sheets are ActiFormCool® (Activa Healthcare), Oxyzyme™ (Archimed) and Iodozyme™ (Archimed); these dressings do not donate water to the wound and, therefore, are less likely to cause maceration, particularly if fitted to the shape of the wound. ActiFormCool will absorb exudate to several times its own size. Oxyzyme and Iodozyme deliver oxygen and iodine to the wound bed.
There are too many amorphous hydrogels on the market to mention but they include Aquaform® hydrogel (Aspen Medical); Cutimed® Gel (BSN medical); GranuGEL® (ConvaTec); INTRASITE GEL™ (Smith & Nephew); NU-GEL® (Systagenix); Octenilin® Wound Gel (Schülke); Purilon Gel (Coloplast); Askina® Gel (B. Braun); and ActivHeal® Hydrogel (Advanced Medical Solutions).
Hydrocolloids
Shallow, sloughy wounds that produce limited amounts of exudate can be treated with a hydrocolloid dressing. These products facilitate autolysis.
Alginates
Sloughy wounds that produce a degree of exudate may be dressed with alginate dressings such as Sorbsan® (Aspen Medical Europe), Tegagen™ (3M), Kaltostat® (ConvaTec) or other gel forming polysaccharide dressings, such as Aquacel® (ConvaTec).
Conclusion
To ensure that a wound healing environment is optimised, debridement of nonviable tissue must be undertaken. Methods used rely on the education and experience of the clinicians, but should be selected based on the wound type and patient needs. Whatever method is selected, slough should not be ignored by the clinician; it is indicative of poor wound bed health and chronicity. Returning wounds – through one, or multiple maintenance episodes of cleansing and debridement – to a healthy, acute state gives the best chance of healing.