Clinicians who routinely manage foot ulceration in individuals with diabetes are aware that infection can be a challenging complication. Infection may not be easily detected, as neuropathy can mask the signs and symptoms and it can be complicated by ischaemia. Diabetic foot infections are reported to develop in 50–60% of ulcers with approximately 20% progressing to moderate or severe infection, which ultimately results in amputation (Edmonds et al, 2021). The risk increases where there is long-standing diabetes with associated advanced neuropathy, joint deformity, peripheral arterial disease and poor concordance with treatment (Lipsky et al, 2021).
Infection develops when bacteria invade soft tissue causing inflammation, this can progress to tissue damage and then spread to underlying tissues, such as muscle, joints and bone (Lipsky et al 2019). Slow or non-healing ulcers increase the risk of osteomyelitis developing as infection progresses through the soft tissue into the cortex of the bone then into the marrow (Gauro et al 2017). The incidence of osteomyelitis is reported to be present in approximately 20% of diabetic foot ulcers (Edmonds et al, 2021).
Many healthcare providers have adopted the recommendations in the Guidelines on the Diagnosis and Treatment of Foot Infection in Persons with Diabetes (Lipsky et al, 2020), which suggest using local or systemic signs and symptoms of infection to detect a soft tissue infection supported with serum biomarkers if necessary. If osteomyelitis is suspected a probe to bone test, plain X-Ray and serum biomarkers should be initially employed with advanced imaging if these are inconclusive.
Infection should be managed with regular debridement of devitalised tissue and appropriate systemic antibiotics which are prescribed from the results of microbiological cultures taken from the wound. This should be delivered as soon as possible to reduce the risk of spread to underlying structures and increase the chance of wound healing. Therapy should be supported by a programme of best practice, which includes vascular assessment, glycaemic control, local wound care and offloading if required (Lipsky et al, 2019).
The use of effective systemic antimicrobial therapy is essential in managing a wound infection in individuals with diabetes (NICE, 2019), but this can be disrupted by the development of antibiotic-related adverse events, such as allergic reactions, further infection with antibiotic-resistant organisms, the risk of developing Clostridium difficile infections and toxicity in certain organs, such as the liver and kidneys (Tamma et al, 2017). Other factors, such as poor vascular perfusion, can limit the availability of antibiotics to the site of infection. These complications can reduce the chance of the infection resolving and consequently the wound healing (Lang, 2000; Turner, 2005).
The concern of increasing antibiotic resistance has led to the development of antimicrobial stewardship (AMS). This recommends a coordinated programme of antimicrobial use to reduce the impact of multi-drug resistant organisms. The use of topical antibiotic agents to treat wound infections has been discouraged because there is limited evidence of effectiveness, and the associated risk of encouraging bacterial resistance (Lipsky et al, 2016). Systemic antimicrobial therapy is the recommended first line treatment for clinically infected wounds. However, adjunctive therapy which uses absorbable calcium sulfate to deliver therapeutic concentrations of antibiotics to the site of infection is increasingly being used for complex foot ulcers in the diabetic individual. This method of delivery using Stimulan is effectively used in orthopaedic surgery where it is shown to inhibit bacterial growth, while avoiding toxic serum concentrations (Maale et al, 2020).
A multidisciplinary group of experts were invited to consider the use of Stimulan in the treatment of infected foot wounds in diabetic individuals, to review the evidence and share their local experience in clinical practice. One senior clinician who had been using it on individuals stated: “Stimulan antibiotic-loaded beads are a wonderful addition in the armoury of treatment of diabetic foot patients in clinical settings and extend our treatment modalities for patients, especially those with multiple intolerances.”
This was undertaken during several virtual discussions which were hosted by Biocomposites Ltd, the company that manufactures and supplies Stimulan. As podiatrists are generally the lead clinicians in managing these individuals, the aim of the discussions was to integrate the evidence with experience and provide them with support and guidance to use this method of antibiotic delivery safely and effectively.
Stimulan to treat foot ulcers in individuals with diabetes
Stimulan may be used within standard wound care undertaken by podiatrists on foot ulcers. Having assessed the wound as infected, the clinician uses baseline bacteriology from the site which is used to inform the antibiotic prescription. This is then mixed with the calcium sulfate paste to form antibiotic-loaded beads (ALB). Following the debridement of devitalised tissue and irrigation, the ALB are placed in a layer on the wound bed (Figures 1&2). This is covered with a secondary dressing to keep them in place (Gauland, 2011) and other therapeutic interventions, such as continued offloading.
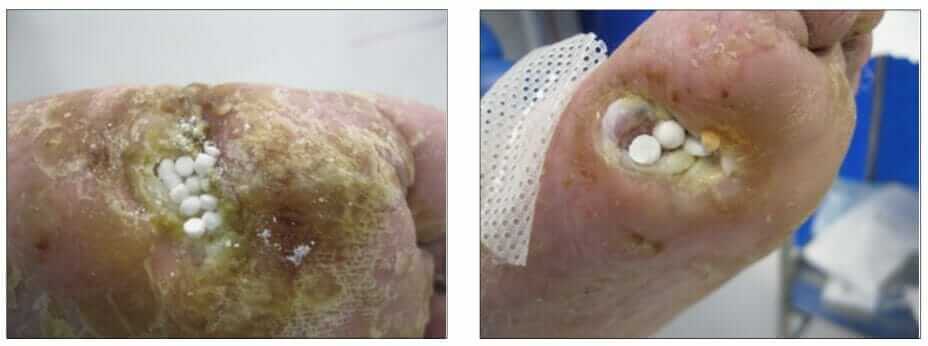
(photographs courtesy of Sam Haycocks).
There is a sustained release of the antibiotics (Aiken et al, 2015; Cooper et al, 2016), and the beads biodegrade over a period of weeks (Oliver et al, 2015). As a result, antimicrobial therapy is delivered directly to the site of infection. This may be of benefit when there is poor local perfusion due to peripheral arterial disease, which limits the availability of antibiotics to the site of infection (Patil et al, 2021).
Clinical evidence for Stimulan
As part of the process the expert group were invited to review the evidence specific to the use of Stimulan on infected foot ulcers in individuals with diabetes. In vitro, it was demonstrated that bacterial growth (including those which were vancomycin and gentamicin resistant) was inhibited, when Stimulan was tested against a mixed bacterial community isolated from debrided tissue from foot wounds of individuals with diabetes (Fletcher et al, 2022). Price et al (2016) investigated the use of Stimulan loaded with tobramycin or gentamicin on a soft tissue wound model of an established biofilm, to assess the susceptibility of bacteria highly tolerant to antibiotics. The results demonstrated a sustained release of antibiotics well above the minimum inhibitory concentrations (MIC) high enough to be effective against a mature biofilm. It was also effective at reducing viable counts for a multi-drug resistant biofilm. In both studies, the authors suggested that the risk of toxicity associated with long-term systemic antibiotic use when treating infected foot ulcers in diabetic individuals may be reduced by using Stimulan as a method of delivery.
The use of Stimulan has been described in several clinical settings. Over a 5-year period, Gauland (2011) evaluated the use of calcium sulfate as a delivery system for vancomycin and gentamicin following surgical debridement on 354 individuals with osteomyelitis. A total of 93.8% (n=303) patients healed following the use of Stimulan and 86.4% (n=279) patients healed without the use of systemic antibiotics. Some 7.4% (n=24) healed with the use of IV antibiotics in addition to Stimulan, 6.2% (n=20) required amputation.
Raglan et al (2018) audited a matched cohort of 23 individuals who had similar ulcer locations and had failed non-surgical treatment. The initial cohort were treated with gentamicin-loaded PMMA beads then the treatment protocol changed to Stimulan loaded with gentamicin. The outcomes were reaudited on a second group of individuals. The use of Stimulan was associated with improved healing times and reduced length of stay. A total of 70% (n=16) of wounds healed within a mean of 6 months (range 2–7) when treated with Stimulan compared to 57% (n=13) who received gentamicin beads. The length of stay with the use of Stimulan was a mean of 7 days (range 1–70) compared to 28 days (range 1–70) with gentamicin-loaded PMMA beads.
In contrast, Dekker et al (2019) retrospectively evaluated a cohort study of 50 patients undergoing surgical debridement of neuropathic foot ulcers for osteomyelitis. Patients were divided into two groups: a group treated with surgical debridement alone (n=13) and a group treated with debridement and implantation of vancomycin and gentamicin impregnated Stimulan beads (n=29). The study concluded that ulcer healing in patients treated with antibiotic-impregnated calcium sulfate beads did not show statistical significance. Healing rates in both groups were similar to those in recent literature. However, all wounds suitable for primary closure were excluded from the study, with all wounds left open to granulate, with calcium sulfate beads used to pack dead space in both bone and soft tissues. The authors noted that this may explain the healing times observed. Also, the study included patients with vascular insufficiency, with 31% of patients having no palpable peripheral pulse. This can also contribute to slow healing rates.
In a retrospective cohort study of 106 patients, Morley et al (2020) evaluated clinical outcomes and costs of those patients who proceeded to surgery in patients with diabetes attending a high-risk foot podiatric surgery community clinic. In 68 out of 70 urgent patients undergoing surgical debridement of devitalised tissue, antibiotic-loaded calcium sulfate (Stimulan) was administered to ensure a high antibiotic dose at the site of infection. A total of 65 of 70 (93%) of these urgent cases were deemed successful, with eradication of infection achieved.
More recently, Morley et al (2021) in a double-centre cohort study retrospectively reviewed the use of Stimulan impregnated with gentamicin and vancomycin on the foot ulcers of 137 individuals with osteomyelitis (n=127) or significant soft tissue infection (n=10) following surgical debridement. The aim of the study was to investigate the time to heal, resolution of infection and duration of postoperative antibiotics.
- In 88.3% of individuals the infection was resolved
- In 22 individuals, the wounds healed without post-operative systemic antibiotics
- Some 82.5% of wounds healed with average healing time of 11.3 weeks. However, it was observed that where the comorbidities of diabetes and peripheral arterial disease were present the healing time was significantly longer (P=< .05).
A retrospective analysis was also undertaken by Patil et al (2021) to observe the outcome of Stimulan when used on 106 individuals following surgical debridement for confirmed osteomyelitis. In 92% (n=98) of individuals there was no recurrence of infection and healing times ranged from 47–64 days. No intravenous antibiotics were administered, and systemic antibiotics were not used after 2-week post op window.
In a small, retrospective, comparative study Qin et al (2019) investigated the use of Stimulan following surgical debridement (n=18) was compared to surgical debridement only (n=28) on the foot wounds of individuals with diabetes. In the outcome of the study there were no significant differences in healing, length of stay or reduction in the use of postoperative antibiotics. The authors attribute this to low subject numbers and short follow up time after treatment. However, it was observed there was no recurrence of infection or amputations reported in the individuals treated with Stimulan. Authors did report that prolonged postoperative leakage was the most common complication, which could be managed with regular dressing changes with all wounds achieving healing.
No adverse effects were reported in any of the studies other than skin maceration which is thought to be due to the dissolution of the antibiotic loaded Stimulan beads.
Outcome
The expert group discussed the use of Stimulan in clinical practice and recognised that podiatrists may experience several issues, especially when working in the community and outside of the multidisciplinary team (MDT). As a result, a tool kit (Figures 3) of guidance documents were developed which could be used by clinicians in their healthcare setting to support them when using Stimulan in clinical practice.

Figure 3. The Document Tool Kit .
The focus of the expert group was to develop a clinical pathway (Figure 4) that podiatrists could use to support them to deliver safe and effective treatment with Stimulan with antibiotics. This involved an in-depth review of using the product within practice, from assessing the suitability of individuals through to monitoring its use, while considering the challenges which may be encountered by clinicians which could restrict its use. The group then considered these issues and using the available research and experience provided a consensus opinion, which could be used to inform practice.
The clinical pathway (below) offers a step-by-step approach to the safe use of Stimulan, which allows the podiatrist to combine this with local protocols for care that are used in practice.

Download the Clinical Pathway
Step 1: Assess suitability for Stimulan with antibiotics
The clinical pathway recommends that as in routine practice, a full thorough individual assessment is essential which includes:
- Identifying any allergy to antibiotics or any other product in use
- Considering the individual’s ability to consent and comply with treatment
- Undertaking baseline investigations as per local protocol. The expert group recommend that no additional blood tests are required before using Stimulan additional to those routinely undertaken when infection is identified, and systemic antibiotics are used.
Vascular status
An assessment of the vascular status of the limb is essential to identify if further intervention could improve the arterial blood supply to the limb. As Stimulan does not depend on local circulation for its delivery, a poor vascular supply should not restrict its use. However, for podiatrists working outside the MDT, the following guidance is recommended:
- When the individual with diabetes presents with an ABPI<0.8mmHg podiatrists working outside the MDT should make a referral for a vascular assessment
- Stimulan can be used with an ABPI<0.4 within an MDT setting that includes a vascular opinion.
Wound assessment
The expert group agreed that the classification system for diagnosing infection recommended by the International Working Group on the Diabetic Foot (Lipsky et al, 2019) is used to identify wound infection. Local protocols for microbiological sampling and detection of bone infection by clinical/radiological investigations should be used. Deep samples with little superficial slough are recommended. No additional tests are required.
An assessment of the wound is essential to ensure that Stimulan can be used effectively within it. The advice of the consensus group is that:
- The wound should be large enough so that a minimum of 1–2 Stimulan beads can be applied onto the wound bed
- The wound bed is sufficiently clear and contains <50% slough. This will ensure that infected viable tissue is in contact with the antibiotics loaded into the Stimulan beads
- For individuals with severe wounds where there is active infection, surgical debridement should be considered prior to use.
The clinical pathway advises that superficial, uninfected new ulcers are not suitable for Stimulan and should, therefore, be treated with conventional wound care as per local protocol. However, if the patient has met the previous criteria, Stimulan can be prescribed.
Step 2. Prescribing and ordering Stimulan
Stimulan is available in two presentations either as calcium sulfate which is mixed with the prescribed antibiotic (vancomycin, gentamicin or tobramycin) to form a paste, which is then introduced into a mould mat to form beads (Stimulan Rapid Cure). Alternatively, the beads are available ready made with Vancomycin and Gentamicin and prescribed as a ‘special’ (Stimulan Antibiotic Beads).
These two presentations of Stimulan are detailed in the clinical pathway to specifically guide the non-medical prescriber.
- Both Stimulan Rapid Cure and Stimulan Antibiotic Beads can be prescribed by a medical practitioner, so should be easily accessed when the individual with diabetes is under the care of an MDT
- Alternatively, if the first prescription is issued by a medical practitioner, this can be added to the individual’s management plan and further prescriptions can be undertaken by non-medical prescribers
- Independent prescriber (IP) podiatrists are not permitted to prescribe ‘specials’. However, IP podiatrists may now mix medicines under the terms of the Statutory Instrument 2013 No 1855. This would allow the mixing of various licensed antibiotics within the Stimulan device, should it be necessary. At present, Stimulan is approved for mixing with vancomycin, gentamicin and tobramycin
Step 3. Application of Stimulan
Within the clinical pathway the application of Stimulan is recommended following debridement. The expert group recommend that:
- As the beads do not absorb exudate, the periwound area is protected from maceration with the use of a barrier film or preparation
- The antibiotic-loaded beads should be packed into the wound filling the cavity. It is important that sufficient beads are used so that they stay in place
- A non-adhesive silicone transparent dressing is used to keep the beads in situ. Additional absorbent secondary dressings and/or retention bandaging can then be applied
- Offloading of the foot should be maintained if necessary.
Step 4. Monitoring
Following the application of Stimulan the frequency of wound monitoring should be dependent on wound progression and infection status. The expert group recommend:
- As in routine practice the wound should be initially inspected at 2–3 days depending on the exudate level and infection status
- If there is an improvement in the wound with a decrease in exudate and reduction in infection, this timescale can be increased
- During monitoring the outer dressings are removed but the beads are left in the wound bed. Any that are dislodged or lost can be replaced
- If the wound is satisfactory the beads are secured in place as previously described, with new outer dressings
- If there are signs of sensitivity or deterioration in or around the wound the beads should be removed
- This process should be repeated supported by local practices for monitoring infection.
Standard Operating Procedure
The content of the Standard Operating Procedure (SOP) includes the information which is included in the clinical pathway. This provides the clinician with a template for the use of Stimulan into which they can import their relevant local guidelines,
Evaluation Process
This consists of two documents which support the clinician in evaluating Stimulan within their clinical practice. A simple guideline advises clinicians on the selection and treatment of appropriate individuals. The evaluation form captures data primarily on wound outcome, infection status and the experience of the clinician and individual being treated. It is recommended that it is completed at the beginning and end of the treatment period with monitoring of the wound recorded by photography at two weekly intervals until the end of the treatment. Information on the safe use of Stimulan is reported by recording the incidence of adverse events.
Discussion
While the use of systemic antibiotics is recommended for individuals with diabetes who have complex infected foot wounds, the use topically is associated with the development of resistant strains of bacteria and, therefore, discouraged (Lipsky et al, 2020). The use of Stimulan as an adjunctive treatment to deliver antibiotics directly to infected tissue, in conjunction with the systemic route may be of benefit, particularly when osteomyelitis is suspected or diagnosed.
A literature search of the use of Stimulan in individuals with diabetes with infected foot ulcers suggests that it may contribute to reduced antibiotic use and improved wound outcomes. However, to support its use in podiatry practice further advice and support was required. This was undertaken by multidisciplinary group of experts who used their expertise and experience to discuss some of the issues associated with the local delivery of antibiotics. As a result, they have developed a toolkit of documents to support clinicians in delivering safe practice when using Stimulan in this way.
The main issues of concern were the perceived risks of antibiotic toxicity and resistance, but evidence from the research has demonstrated that the risk is very limited (Livio et al, 2014; Wahl et al, 2017; Haque et al, 2018). This research along with the experience of the experts in the group can reassure clinicians that Stimulan is safe to use within the manufacturer’s recommendations.
The main side-effect of using Stimulan reported in the literature review was the increase in exudate, which some investigators found problematic to manage. Despite this, in most studies the time to healing was decreased. Patil et al (2021) suggested reducing the number of beads applied to the wound, although this is not generally recommended as it increases the risk of them being dislodged from the wound bed. The risk of developing periwound maceration should not be a barrier to using Stimulan given there are several products available to prevent this and manage exudate effectively. This was addressed in the clinical pathway and recommendations made to prevent this complication.
Most investigators of the clinical studies published to date concluded that the use of Stimulan following debridement may improve the outcomes of foot ulcers in individuals with diabetes. As a result, the future opportunities for Stimulan with antibiotics were discussed. While the clinical studies to date generally include enough subjects, these were retrospective and not controlled. While this study methodology is positive in that it provides real-life data on use, the evidence from randomised controlled trials would be more conclusive.
Conclusion
Preventing and managing infection in individuals with diabetes who develop foot ulcers is challenging for clinicians. Stimulan offers a real opportunity to deliver relevant and effective antibiotics within a programme of care to the wound bed within a framework of best practice in foot ulcer management. Further research is needed to investigate whether this method of delivery can improve healing rates within these wounds and potentially reduce the need for systemic antibiotics.
Appendices
Appendix 1 Standard Operating Procedure
Appendix 2 Evaluation guidelines
Appendix 3 Evaluation form
Clinical pathway – Download here




