Diabetes is an extreme and severe health burden, associated with comorbidities that include myocardial infarction, stroke, renal failure, blindness and risk of lower limb amputation (Wang et al, 2024). Diabetes foot ulcers (DFUs) are a debilitating manifestation of sub-optimal glycaemic control and diabetes. The presence of DFUs can lead to increased length of hospitalisation and increased demands on healthcare. Approximately 15% of individuals diagnosed with diabetes will develop a DFU, of which 14–24% will require amputation (Raja et al, 2023). The management of individuals with high-risk foot disease will require routine medical assessment, medical care and self-care. Over 1 million individuals worldwide undergo lower limb amputation associated with diabetes annually, and its impact imposes severe economic burdens (Wang et al, 2024).
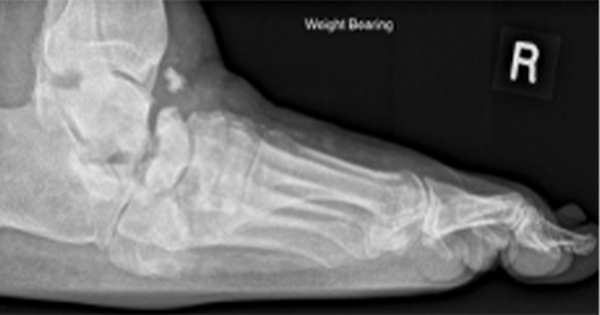
Osteomyelitis is an inflammatory bone disease characterised by severe inflammation, vasculature impairment and bone destruction. Infective microorganisms will trigger an immune response in the form of inflammatory cytokines and enzymes that break down infected tissue. Intercellular spaces can be filled with leukocytes and bacterial cells in the form of purulence, which can lead to abscess formation and bone necrosis (Kavanagh et al, 2018). Osteomyelitis should be considered as a potential complication of infected, deep or chronic DFUs, particularly where the ulceration overlies a bony prominence (Lipsky et al, 2012).
Clinical awareness of factors that increase the risk of osteomyelitis and other types of diabetes foot infection (DFI) is paramount and can be cured if appropriately managed. Risk factors will include positive probe-to-bone test, non-healing DFU, traumatic wound, presence of peripheral vascular disease, previous lower-extremity amputation, loss of protective sensation, and renal insufficiency (Lipsky et al, 2012). If left untreated, such risk factors can consequently lead to exacerbation of the skin, microbial infection and impediment of the healing of ulceration (Wang et al, 2024).
DFI management is an incredibly complex balance of surgical intervention, conservative management and antimicrobial therapy (AMT) (Kavanagh et al, 2018). Podiatrists play a crucial role in the sharp debridement of infected bone and soft tissue of DFUs (Haidar et al, 2010). This is fundamental in the management of osteomyelitis. Successful treatment will depend on accomplished debridement of infected de-vitalised tissue, and surgical resection of bone necrosis (Kavanagh et al, 2018). Probe-to-bone testing, serial plain radiographs, bone culture and histology will help to support either diagnosis or exclusion of infection and determine definitive therapy. Such diagnostics, including bone biopsy, will very often be obtained through the podiatrist as the treating clinician (Lipsky et al, 2012).
Prescribing antimicrobial therapy for DFIs in podiatry
Guidance targeting the duration of AMT for DFIs is varied. NICE guidelines (2019) advise that first-line oral therapy is started as soon as possible if there is suspicion of foot osteomyelitis. Conversely, many papers have suggested debridement of bone necrosis prior to AMT (Kavanagh et al, 2018). Antimicrobial resistance is a global threat to public health and the economy (Fitzpatrick et al, 2022). Therefore, excessive and inappropriate antimicrobial use can have negative effects on patient care, the health system and society. Uckay et al (2015) recommended that AMT is not necessary in most presumed uninfected ulcers where appropriate wound care is carried out, and that clinically uninfected DFUs should not be treated with antibiotics. Lipsky et al (2012) suggest up to a 2-week antimicrobial course for soft tissue DFIs or up to three weeks for more severe infections. The choice to prescribe prolonged antibiotics for osteomyelitis has been traditionally adopted, but there is limited evidence. Considering the advancements in surgical techniques, this poses the question of whether traditional methods of ‘prolonged AMT’ are still effective or should be reconsidered (Haidar et al, 2010).
The podiatrist has a crucial role as the primary treating clinician to coordinate care amongst consulting specialists. Early minor-surgical intervention combined with antibiotics for deep DFIs may be necessary to reduce major amputation as opposed to AMT alone. Podiatrists are advised to refer to a vascular surgeon for patients with moderate or severe DFIs in consideration of revascularisation during the early stages of the foot complaint, particularly if ischemia is a complication (Lipsky et al, 2012). In some cases, surgery is not possible, and so long-term AMT is the most suitable option (Besal et al, 2023).
Prescribing practice in podiatry has mainly contributed toward a broadened scope of podiatry, autonomous practice, and improved outcomes for patients (Fitzpatrick et al, 2022). Podiatrist independent prescribers at the Royal Free Hospital in London refer to the local adult antimicrobial guidance, known as the ‘micro-guide’ for the prescribing of medicines, including antimicrobial therapy for the treatment of diabetic skin and soft tissue infection (Adult Antimicrobial Micro guide 2024). The micro-guide advice is to refer all DFUs with visible bone, probe to bone and/or confirmed osteomyelitis to the infectious diseases/microbiology team.
Aims and objectives of the study
A clinical audit was carried out by podiatrist independent prescribers within the multidisciplinary foot clinic at the Royal Free Hospital in London, which studied the antimicrobial prescribing pathway for the treatment of DFIs. The objectives of the audit were:
- To determine the level of autonomy of the podiatry team as independent prescribers, and the number of prescriptions written by the podiatry team.
- To indicate which antimicrobials are prescribed to patients as first-line treatment for DFIs.
- To indicate how many patients are referred to infectious diseases following first-line prescription for treatment of DFIs.
- To review patient outcomes following antimicrobial therapy for DFIs, study the cohort of patients that show improvement and decisions made.
- To evaluate infectious diseases in decision-making for patients showing signs of improvement following an antimicrobial course for treatment of DFIs.
Methodology
Eighty-two patients were prescribed first-line AMT for a presenting acute foot complaint for 6 weeks. Patients within the audit were seen on the wards, the diabetes foot clinic, or in the ambulatory acute foot service in the emergency department [Figure 1]. Data were collected at the point of the first antimicrobial prescription, and for consecutive prescriptions. Data were also collected on final patient outcomes [Figure 2–4].
Key findings
Findings from the study showed 38 out of 82 (68%) antibiotic prescriptions were written in the outpatient diabetes foot clinic at the Royal Free Hospital [Figure 1]. Outpatient diabetes foot clinic and ambulatory acute foot clinic combined showed 56% of prescriptions for AMT were written by podiatrist independent prescribers in the podiatry team at the Royal Free Hospital [Figure 5]. No prescriptions were written by the podiatry team for inpatients requiring AMT.
AMT was prescribed for the following reasons: suspected osteomyelitis, confirmed osteomyelitis, cellulitis or soft tissue infection [Figure 6]. Co-amoxiclav was prescribed to 61% of patients as a first-line antibiotic [Figure 7]. It was prescribed in accordance with guidance from the micro-guide for confirmed or suspected diabetes foot osteomyelitis. 49% of patients were referred to infectious diseases following first-line prescription [Figure 8]. This was reflective of the number of patients with confirmed or suspected osteomyelitis who had ulcers exposed or probing to bone [Figure 6].
Following the first course of AMT, data were collected to determine the outcome of treatment [Figure 2]. 78 out of 82 subject outcomes were documented from the first review following the first course of AMT. Out of the four remaining patients, one patient was discharged with antibiotics, one patient deceased, and two patient outcomes are unknown. Out of the entirety of the study, 37% of antibiotics were stopped after the first review [Figure 3]. Conclusively, 96% of patients had either improved or resolved DFIs, which means that antibiotics did not need to be changed or extended if the presenting complaint had improved [Figure 4].
Implementation of antimicrobial prescribing pathway
As a result of this study, a new antimicrobial prescribing pathway was proposed for patients presenting with diabetic foot-related complaints or infection [Figure 9]. The new pathway aimed to give podiatrists independent prescribers more autonomy in the prescribing of medicines for patients with less complex and stable wounds, thus reducing the burdens of multiple referrals to infectious diseases or microbiology departments. Prior to implementation, the proposed pathway underwent a trial period within the diabetes foot clinic and ambulatory acute footcare clinic at the Royal Free Hospital, London. To maintain patient safety and safety netting of complex cases, guidance was included for podiatrist independent prescribers to follow in the pathway. These included:
- Clear indication of when AMT is deemed appropriate
- Clear indication of when to refer to the local ‘micro-guide’ for prescription advice
- Clear indication of when to refer or involve infectious diseases teams in the care of the patient or decisions made
- Further guidance at each point of patient contact, and at what stages to carry out baseline investigations, including blood tests, microbiological and radiological findings
- Guidance on when to query a suspect or confirmed osteomyelitis
- Clear multidisciplinary escalation and safety-netting processes, including joint MDTs and emergency bleep contacts.
It was important to evaluate the number of referrals to infectious diseases/microbiology departments prior to and after the implementation of the programme. Referrals were audited eight weeks prior to implementation and eight weeks following implementation. Results showed 29 patients were referred in total to the infectious diseases department for a formal consultation. Results showed a significant reduction of referrals from 72% of patients referred in the first 8 weeks pre-implementation of the pathway, to 28% referred in the second 8 weeks post-implementation. Reducing referrals to the infectious diseases department by 44% received positive feedback. This contributed toward reduced burdens on the medical teams, increased interdisciplinary approaches to patient care, and increased autonomy for podiatrist independent prescribers [Table 1].
Conclusion
Podiatrists who work within diabetes foot clinics are the primary treating clinicians for such patient groups and play a crucial role in the management of DFIs and osteomyelitis. Therefore, podiatrists who work within diabetes foot clinics should be inspired to pursue prescribing qualifications. Podiatrists who do prescribe independently are encouraged to participate in antimicrobial stewardship programmes and prescriber networks to ensure safe and appropriate care of prescriptions for their patient groups. This will in turn support prevention of overuse and misuse of AMT, and reduce inappropriate resistance patterns, infection incidence and hospital length of stay (Nathwani et al, 2019; Royal College of Podiatry, 2018).
Prevalence of antimicrobial resistance and adverse effects of AMT emphasise a significant need for alternative approaches to managing the occurrence of DFIs (Fitzpatrick et al, 2022). Furthermore, the duration of AMT for DFIs requires more research. The quality of evidence surrounding short-term vs long-term antimicrobial therapy for osteomyelitis is low and inconsistent. There is also inconsistency in data, whereby high-risk complex patient cases, patients with comorbidities, and cases where pathogens are not identified are often excluded. Patients will continue to be treated on a case-by-case basis by experienced clinicians, but standardisation of guidance based on evidence and further prospective studies is needed to determine optimal duration of antimicrobial therapy (Besal et al, 2023; Haidar et al, 2010).
Results of this study demonstrated a 44% reduction of referrals to the infectious diseases or microbiology teams. This highlighted the autonomy of podiatrist independent prescribers within diabetes foot clinics. Having said this, as autonomous prescribing practice prevails in podiatry, certain challenges associated with broadening the scope of practice will persist. This includes limitations to prescribe controlled drugs, and inability to fully utilise independent prescribing skillsets within current legislative frameworks (Fitzpatrick et al, 2022). Interestingly, within this study of 82 participants, 0% of inpatient antimicrobial therapy was prescribed by the podiatrists. This can pose issues in the ability of podiatrist independent prescribers to fully manage complex DFIs and post-surgical cases, which can inevitably place increased burden on physicians (Fitzpatrick et al, 2022).
Conclusively, to enhance diabetes foot care within clinical settings and decrease amputation rates, management plans will require prompt diagnosis and classification of infection severity (Lipsky et al, 2012). The balance of achieving bacterial eradication, minimising antimicrobial resistance and adverse effects, with the consideration of financial healthcare pressures, will require a holistic interdisciplinary approach to treatment. Podiatrists are the foundation of diabetes foot care, whereby AMTs play a crucial role in its management. Therefore, advancement in diabetes foot care should be led by the increasing availability of podiatrists and broadening of the scope of practice (Besal et al, 2023; Uckay et al, 2015).