There is no register of diabetic foot ulcers in England, so there is uncertainty as to patient numbers. Recent snapshots audits in Scotland suggest that 2–2.5% of people with diabetes have ulcers at any given time (Leese et al, 2011; personal communication, Graham Leese). If prevalence is similar in England, we can estimate that, in 2014–15, 58,000 people had active ulcers on any given day. The number experiencing ulceration over the course of a year is likely to be considerably higher. By comparison, approximately 46,000 people in England were diagnosed with breast cancer and 40,000 with prostate cancer in 2015 (Office for National Statistics, 2015).
Approximately 7,000 people started renal replacement therapy for end-stage renal disease in that year (Byrne et al, 2017).
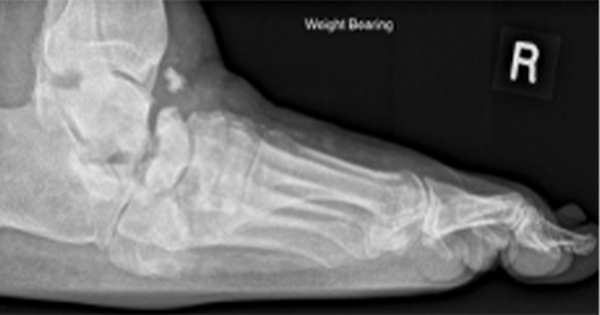
Many ulcers persist for months; some never heal, and some lead to amputation. In England, there are around 9,000 lower-limb amputations in people with diabetes annually (Public Health England, 2019). The risk of a person with diabetes undergoing a lower-extremity amputation has been estimated at 23 times that of someone without diabetes (Holman et al, 2012). As diabetes prevalence increases, so too does the number of amputations; there were almost 14% more diabetes-related amputations in England in 2015–18 than in 2012–15.
Both ulcers and amputations substantially reduce health-related quality of life. EQ-5D scores, which range from 0 (death) to 1 (perfect health), have been estimated at 0.44 for people with diabetic foot ulcers and 0.31 for those who have undergone major amputation (Ragnarson Tennvall et al, 2000). These scores are lower than those recorded in other studies for people with breast cancer, prostate cancer (Pickard et al, 2007) or end-stage renal disease requiring haemodialysis (Wasserfallen et al, 2004).
Mortality rates are high after both ulcers and amputations. Five-year mortality after first major amputation has been estimated at 68%–79% (Icks et al, 2011; Ikonen et al, 2010), comparable with the rate for people with diabetes starting renal replacement therapy (68%) (Byrne et al, 2017) or after a first stroke (44%) (Icks et al, 2012). Five-year mortality after diabetic foot ulcer has been estimated at 40% (Jupiter et al, 2016) and the hazard ratio for death at 2.48, after adjustment for other risks (Walsh et al, 2016).
In addition to these human costs, the financial costs to the NHS are large and increasing. A recent study estimated the cost of healthcare for ulceration and amputation in diabetes in England in 2014–15 at £837m–£962m (Kerr et al, 2019). This is equivalent to almost £1 in every £100 spent by the NHS in England, and is higher than estimated NHS expenditure on breast, prostate, and lung cancers combined (NHS England, 2014).
Of these diabetic foot costs, nine tenths were for ulcers. While amputation unit costs are high, the far greater incidence of ulceration produces higher aggregate costs. The cost of a week of care for a severe ulcer (SINBAD score ≥3) was more than three times the cost of care for a less severe ulcer. These costs were incurred across inpatient, community, outpatient and primary settings. For inpatients, ulceration was associated with a length of stay 8.04 days longer (95% confidence interval 7.65 to 8.42) than that for diabetes admissions without ulceration. However, more than half the costs were incurred in community and outpatient settings.
It is likely that there is scope for considerable improvement in care in many parts of England. The incidence of major amputation varies sevenfold across the country, after adjustment for age and ethnicity (Jeffcoate et al, 2017). There is also substantial geographic variation in service provision, and delays in assessment are associated with increased ulcer severity and longer ulcer duration. The proportion of ulcers healed at 24 weeks after expert assessment varies by 40% across providers (NHS Digital, 2019).
It is known that early access to specialist care can reduce ulcer duration, improve healing rates, and lower amputation rates (Ince et al, 2007; Krishnan et al, 2008; Canavan et al, 2008). In recent years, some areas of England have systematically improved services and reported significantly improved outcomes (Paisey et al, 2018).
If the NHS were to reduce the prevalence of diabetic foot ulcers in England by one-third, the gross annual saving would be more than £250m. Simultaneously reducing the proportion of severe ulcers would reduce costs still further. It is important to note that these are gross savings; they do not take account of additional costs incurred in service improvement.
As we have seen, much diabetic foot care is provided in community settings, such as podiatry and district nursing. For these services, patient-level and diagnosis-specific data are generally not available in England, and block contracts are frequently used for funding. As a result, commissioners are often unaware of how resources are used. The absence of routine data in this area, and the resulting ignorance of resource use and costs, arguably contribute to the relative neglect of this serious and common complication of diabetes.
High and rising costs are also seen in other parts of the world. While direct comparison of costs across countries is challenging owing to differences in care and cost structures, as well as in study design and demographic characteristics, recent systematic reviews indicate increasing costs for diabetic foot disease in several health economies (Petrakis et al, 2017; Tchero et al, 2018). It is hoped that by exploring the hidden costs of foot care, and of severe and prolonged ulceration in particular, this article will support decision-making on diabetic foot care in England, and also more widely.