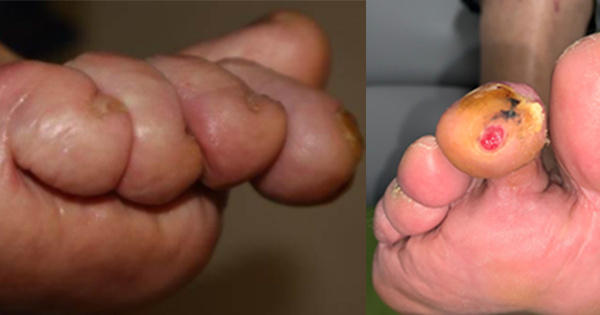
Diabetic foot ulcers (DFUs) are a frequent concern for both physicians and plastic surgeons, often requiring multidisciplinary care. Defined as a full-thickness skin defect between the ankle and toes in patients with diabetes, DFU complicated by infection significantly increases the risk of necrosis, gangrene, and amputation (Edo et al, 2013). Diagnosis of diabetic foot infection may be clinical or microbiological, with tissue biopsy considered the gold standard for culture. However, swab techniques are often criticised for isolating contaminants (Gerding, 1995). Levine et al (1976) proposed a standardised swab method that may offer a reliable, less invasive alternative for microbiological sampling. Hallboom et al (2019) in The Netherlands had documented that LST yields comparable results to deep tissue biopsy technique (DTBT).
Materials and methods
This prospective, comparative, cross-sectional study was conducted in a level-one trauma and regional burns centre in southeastern Nigeria. The hospital provides orthopaedic, plastic and reconstructive surgery services. Adult patients with DFUs (Wagner grades I–III) of <6 months’ duration were recruited from the surgery outpatient, adult emergency and surgical wards between April 2016 and March 2017, following approval in accordance with the ethical guidelines of the Declaration of Helsinki. Patients were eligible for participation if their wound was suitable for both wound biopsy and wound swab, thus excluding patients with a wound bed completely consisting of exposed bone or tendon, wound size <5 mm, wound with a necrotic or sloughy floor and patients who used antibiotics in the 5 days prior to study participation.
Wound sampling was performed after cleaning and topical anaesthesia. The Levine swab technique (LST) involved rotating a sterile swab over a 1 cm² area of granulation tissue under firm pressure for 5 seconds. The deep tissue biopsy technique involves obtaining a full-thickness tissue biopsy from the same site and placing it in sterile saline. Haemostasis was achieved before dressing. To prevent contamination, the skin around the wound was cleansed with chlorhexidine solution and alcohol prior to wound biopsy.
Samples were processed within 15 minutes of collection. Aerobic cultures were incubated on blood and MacConkey agar at 35°C for 24–48 hours. Isolates were confirmed using standard microbiological methods, and antibiotic susceptibility was determined by disc diffusion per National Committee for Clinical Laboratory Standards guidelines (Kiehlbauch et al, 2000). Microbial load was expressed as colony-forming units. Colony-forming units involve diluting, plating, and counting visible colonies of viable bacteria on a nutrient agar plate (Nelson et al, 2013).
Results
A total of 70 samples were obtained from patients who met the inclusion criteria. The demographic and clinical characteristics of these patients are presented in Table 1. Clinical signs of infection were present in 100% of the wounds. Culture results from a wound biopsy yielded 60 isolates versus 61 isolates when culture results from a wound swab were provided. Gram-negative organisms were the predominant organisms (70%) for both techniques. Coliforms (Citrobacter, Enterobacter) were most frequent (51%). Staphylococcus aureus was the leading Gram-positive (LST: 10 [14.3%]; DTBT: 4 [5.8%]). Concordance between methods occurred in 47 (78.3%) wound samples. Differences in organisms identified were significant for S. aureus (p=0.04) and E. coli (p=0.01), and borderline for Streptococcus pyogenes (p=0.08) [Table 2].
Discussion
In this study, DFUs occurred slightly more often in the right lower limb (51%), suggesting no significant limb predilection, consistent with the systemic nature of diabetes. Most patients presented late, with 80% exhibiting Wagner grade III ulcers, similar to findings in Ibadan (Adeleye, 2005), Benue (Akaa et al, 2017) and Zaria (Danmusa et al, 2016) (Nigeria). Delayed presentation may be due to peripheral neuropathy and inadequate pre-hospital care. An abnormal ankle–brachial index was observed in 23% of patients, likely reflecting peripheral arterial disease, a known risk factor for diabetic foot infections (Lavery et al, 2006).
Chronicity of ulcers influenced microbial colonisation, with moderate to heavy growth observed in chronic wounds, consistent with prior studies showing that chronic wounds harbor bacteria, often from skin flora, supplemented by opportunistic pathogens (Lipsky et al, 2012). Comparison of LST and DTBT sampling showed similar identification of pathogens, supporting previous reports from Israel, Saudi Arabia and India (Slater et al, 2004). LST isolated more Gram-positive organisms (p=0.003), while coliforms were the most common overall, consistent with studies in Saudi Arabia. Species concordance between LST and DTBT was 66.8% (p=0.01), with significant differences for S. aureus (p=0.0001) and E. coli (p=0.01), comparable to findings from the USA, UK and Saudi Arabia (Bozkurt et al, 2011; Wren, 1980).
The microbial load differed significantly between techniques, with LST yielding fewer isolates per sample (4.27 vs. 4.91, p=0.001) (Wren, 1980) In 91% of samples, the antibiotic sensitivity results were the same (Bozkurt et al, 2011). DTBT identified a few additional cases (8.3%) in which bacteria were sensitive to more antibiotics, whereas LST identified very few (0.9%). The small differences between the tests were not statistically significant. This suggests that antibiotic selection would be appropriate in most patients based solely on LST results (Pellizzer et al, 2001). This study found that LST was cheaper, less painful and easier to carry out, yet still effective in detecting the responsible pathogens and informing antibiotic choices, consistent with studies from Germany and the UK (Fleck et al, 2006; Stott, 2007). Although DTBT remains the gold standard, this study observed that it carries higher risks of tissue trauma, bleeding, and pain, similar to findings by Ademola et al (2013) in Ibadan (Nigeria).
In 85.7% of all wounds, the isolation of microorganisms did not differ between culture results obtained from the wound biopsy and wound swab [Table 2]. Assessments did not differ significantly when specific microorganisms were cultured. When the biopsy technique was compared with the LST for identifying organisms, they identified similar organisms 86% of the time (ranging from 85.7% to 87.1%), supporting the use of LST as a reliable and practical alternative for diagnosing and guiding treatment of diabetic foot infections.
Limitations of the study
The limitations of this study include its single-center design and the absence of anaerobic culture testing, which may restrict the broader applicability of the results.
Conclusion
Our study demonstrates the reliability of the LST for wound sampling in the diagnosis and management of diabetic foot infections. The results provide strong evidence supporting its use as an effective method for obtaining accurate microbiological samples, which serves as a guide to antibiotic choice. Given its simplicity, cost-effectiveness, and reproducibility, the LST could be integrated into routine clinical practice to improve diagnostic accuracy and treatment outcomes. Future studies should further explore its comparative effectiveness against other swabbing methods and its role in monitoring treatment response over time. We also recommend a multicentre study that includes cultures of anaerobic organisms.