Charcot neuroarthropathy (CN) is a relatively under-recognised condition that affects joints – most commonly in the feet, but also in the hands and other locations. It is characterised by progressive damage and destruction of joints, bones and surrounding soft tissues, and occurs primarily in patients with peripheral neuropathy. CN is most frequently associated with diabetic neuropathy of the foot, where inflammation of soft tissues initiates a destructive process that can ultimately lead to joint collapse and deformity.
The consequences of CN can be severe, including structural deformity, ulceration, infection, and in some cases, limb amputation. These complications significantly reduce life expectancy. Diacogiogris et al (2021) highlighted the gravity of this condition, and yet underdiagnosis remains widespread, largely due to limited clinical awareness. Armstrong and Lavery (1998) reported a 13% incidence of CN among patients with diabetic neuropathy, while Lomax and Jones (2007) observed incidence rates ranging from 10.8 to 27.4 per 10,000 individuals with diabetes over a 10-year period.
Clinically, CN typically presents as a red, hot and swollen foot with the hallmarks of an inflammatory response alongside ongoing soft tissue and osseous destruction. However, we propose the existence of a much earlier stage of the condition, marked by a subtle and easily overlooked presentation. The long-established Eichenholtz classification system has been widely used to aid in diagnosis and staging of CN (Shibata et al, 1990). Nonetheless, early-stage CN remains particularly challenging to detect due to the absence of clear clinical or radiological signs, which likely contributes to ongoing underdiagnosis.
The economic burden is substantial: diabetic foot complications, including CN, cost the NHS an estimated £1.2 billion in 2024 (Department of Health and Social Care, 2024). Furthermore, the prognosis for patients undergoing a below-knee amputation due to diabetic complications is dire, with reported life expectancy as low as three years (Beyaz et al, 2017). Given that CN is a significant contributor to such outcomes, this review is driven by the urgent need to improve early recognition and intervention strategies for CN.
Aim
CN is traditionally recognised as a condition marked by inflammation, typically presenting in the early (Stage 0) phase with a red, hot, swollen foot, and subtle or absent radiographic changes. However, emerging clinical observations including those drawn from the two case studies presented in this article suggest that an earlier, non-inflammatory stage may exist. This pre-inflammatory phase is characterised by neuropathic symptoms such as discomfort or altered sensation in the absence of overt inflammation or visible structural change. Such a stage is not currently acknowledged within mainstream diagnostic frameworks, including the Eichenholtz classification or existing clinical guidelines (National Institute for Health and Care Excellence [NICE], 2019; International Working Group on the Diabetic Foot [IWGDF], 2023).
The primary aim of this article is to explore the plausibility and clinical relevance of a non-inflammatory stage of CN by integrating real-world clinical observations with a systematic review of the literature. Specifically, it seeks to determine whether there is evidence to support the existence of a prodromal phase prior to the inflammatory presentation and whether current clinical assessments and diagnostic pathways are sufficient to detect this phase.
To achieve this, two aims are addressed in parallel:
1. Clinical objective:
To examine two patient case studies that demonstrate neuropathic symptoms prior to any visible inflammation, swelling, or temperature change, thereby suggesting the presence of a non-inflammatory stage. These cases challenge the assumption that inflammation is always the initial manifestation of CN.
2. Research objective:
To conduct a targeted literature review using a structured methodology and critical appraisal tools (CASP) to identify, evaluate, and synthesise existing evidence that may support or refute the existence of this non-inflammatory stage. The review also examines the robustness of the literature with respect to diagnostic criteria, clinical assessment tools, and imaging modalities used in the early detection of CN. In total 43 papers were considered. After assessment of inclusion criteria, six papers were identified for inclusion. The remaining 37 papers were reviewed and included for discussion and reference.
The findings of this review may have significant implications for clinical practice. If a non-inflammatory stage is substantiated, this could explain the high rates of delayed or missed diagnosis of early CN and underscore the need to revise existing diagnostic pathways. Improved understanding of early symptomatology – beyond classic inflammation – could enable earlier recognition, prompt intervention, and potentially reduce the risk of irreversible joint destruction, foot deformity, ulceration, infection, and ultimately, lower-limb amputation.
By combining literature analysis with clinical evidence, this article aims to broaden the current understanding of CN progression and stimulate further research into early diagnostic markers. Ultimately, it advocates for greater clinical vigilance and an updated diagnostic framework that includes a potential non-inflammatory prodromal stage of CN.
Case study 1: Non-inflammatory onset of Charcot neuroarthropathy – a delayed diagnosis
Patient summary
A 67-year-old man with a 15-year history of long-standing, poorly controlled type 2 diabetes (HbA1c currently 76 mmol/l; historical range 65–112 mmol/l) presented with multiple diabetes-related complications. He had dense peripheral neuropathy with complete loss of sensation to the 10 g monofilament extending above the ankle, early-stage diabetic retinopathy, stage 3 chronic kidney disease,and established cardiovascular disease.
Initial presentation
The patient attended a GP appointment reporting pain in the midfoot region of his right foot. At the time, there were no signs of swelling, erythema or temperature difference between the feet. The GP’s clinical notes confirmed this.
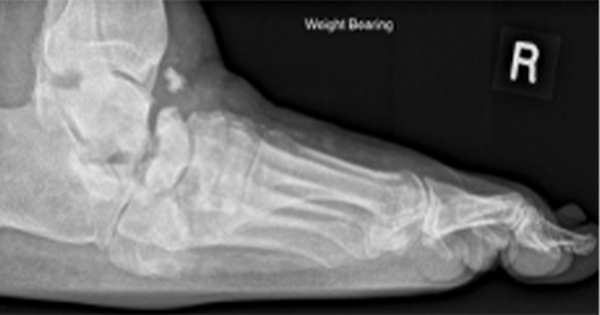
Based on the symptoms, the patient was diagnosed with gouty arthritis and managed accordingly. An initial foot X-ray showed no abnormalities, including no evidence of osteoarthritis, gout or Charcot changes (Figure 1).
Progression
Over the next 6 months, the patient continued to experience persistent midfoot pain with no improvement. A second consultation led to a revised diagnosis of osteoarthritis. No further imaging was performed at that time. NSAIDs were prescribed but failed to alleviate the pain. Despite documented peripheral neuropathy, the possibility of early-stage CN was not considered.
The patient remained mobile, bearing weight on the affected foot, and over the next 2–3 years developed gradual postural changes, intermittent swelling, and ongoing pain. Four years after the initial presentation, the patient was referred by the GP to a community podiatrist and then to the diabetic foot clinic, where he was finally diagnosed with stage 1 CN according to the Eichenholtz classification.
Clinical imaging and findings
The initial X-ray showed no pathology (Figure 1). However, follow-up X-rays taken 4 years later (Figures 2 and 3) demonstrated significant destructive changes in the midfoot and rearfoot, including collapse of the medial longitudinal arch and soft tissue swelling which are hallmarks of active CN, consistent with Eichenholtz stage 1 (Diacogiorgis et al, 2021).
Case study 2: MRI confirmation of early Charcot without inflammatory signs
Patient summary
A 58-year-old woman with an 11-year history of poorly controlled type 2 diabetes (current HbA1c 70 mmol/l; historical range 67–115 mmol/l) and established peripheral neuropathy attended the outpatient diabetic foot clinic for a routine follow-up. Neurological assessment revealed dense loss of sensation to the 10 g monofilament over the forefoot and midfoot, with patchy preservation of sensation in the rearfoot. She reported a progressive onset of midfoot pain in her left foot, worsening over several weeks. No history of trauma was recalled.
Clinical assessment
On examination, there were no signs of swelling, inflammation, or temperature difference between the left and right foot. Foot posture was also symmetrical. Musculoskeletal assessment included the piano key test, targeting the tarsometatarsal (TMT) joints (Rossi and Wallace, 2021). Pain was elicited over the first and second TMT joints on the left foot, but not on the right.
Further firm palpation of the base of thefirst and second metatarsals and adjacent cuneiforms produced pain on the left side only. These tests revealed localised discomfort despite the absence of classical signs of Charcot, as defined by IWGDF (2023) and NICE (2019), which emphasise the presence of redness, warmth, swelling or deformity.
An initial X-ray revealed no definitive osseous or joint changes consistent with Charcot (Figures 4–6).
Intervention and diagnosis
Based on clinical suspicion and pain localised to specific midfoot joints in the absence of inflammation, a working diagnosis of early-stage Charcot was made. The patient was offloaded and casted to protect the foot and prevent further joint collapse. An urgent MRI was requested, although the scan was delayed by 6 weeks due to wait times.
The MRI revealed bone marrow oedema and cortical irregularity at the first and second TMT joints—findings consistent with early CN (IWGDF, 2023). These findings validated the clinical diagnosis made, despite the lack of traditional inflammatory signs.
Discussion
Lack of awareness
A consistent finding across the literature is a significant lack of clinical awareness of Charcot neuroarthropathy (CN), both in early and late stages. Dardari (2020) and Diacogiorgis et al (2021) highlight that CN remains underdiagnosed, particularly outside diabetic foot teams. Myerson et al (1994) reported that 25% of patients referred for CN had not received a correct diagnosis from the referring institution. This underdiagnosis contributes to delayed offloading and increases the risk of deformity and amputation.
Yablon et al (2010) and Vopat et al (2018) reiterate the lack of awareness among non-specialist clinicians, contributing to widespread underreporting. Armstrong and Lavery (1998) estimate the incidence of CN in diabetic patients with peripheral neuropathy could be as high as 13%. Recently, Wukich et al (2024) suggested that we stop considering that it is a rare incident but a more common one that occurs in patients with diabetes. Diacogiogis et al (2021) emphasised the need for robust clinical pathways in hospitals to raise awareness and support timely diagnosis and treatment, advocating urgent referral to specialists upon suspicion of CN.
Criteria for clinical suspicion
Maden and Pai (2013), Milne at al (2013) and Rosskopf et al (2019) agree on the importance of a structured approach to clinical suspicion. Classical presentations include inflammation (swelling, redness, pain) and a temperature difference of ≥2°C (Milne et al, 2013). However, atypical early-stage presentations with low-grade or absent inflammation have been documented by Jones et al (2012), which Maden and Pai (2013) refer to as “cold acute Charcot.” These presentations can occur in patients with impaired inflammatory responses due to diabetic immunopathy.
Rajbhandari et al (2002) emphasises the variability in neuropathic symptoms, with some patients experiencing sensation or pain in previously insensate feet. Petrova (2007) supports this, finding that systemic inflammatory markers, such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and white blood cells (WBC), may not be elevated. These subtle or atypical signs complicate diagnosis and raise questions about whether a new “pre-stage 0” should be added to the Eichenholtz classification system.
Diagnosis
All papers reviewed identified early-stage CN diagnosis as particularly challenging due to its subtle and variable clinical presentation. Maden and Pai (2013) and Milne et al (2013) highlighted that plain film X-rays are often ineffective early on due to low sensitivity. A thorough clinical examination and history are vital, though not always practised comprehensively. Maden and Pai (2013) also noted a gap in the literature regarding robust musculoskeletal assessment, such as joint mobilisation and palpation for crepitus or localised tenderness.
Clinicians are encouraged to maintain a high index of suspicion when a neuropathic foot presents with unexplained swelling, pain, or increased temperature, even if inflammation appears minimal. O’Loughlin et al (2017) found that only 38% of early-stage CN patients had an MRI ordered, despite guideline recommendations that early imaging is crucial.
Imaging
MRI is unanimously recognised as the most effective imaging modality for early-stage CN. It can detect bone marrow oedema and soft tissue inflammation on fat-suppressed sequences. However, differentiating CN from osteomyelitis remains a challenge (Botek et al, 2010). Contrast-enhanced MRI can improve specificity by identifying subcortical inflammation (Rosskopf et al, 2019).
Ahluwalia et al (2020) and Dardari (2020) explore advanced imaging like SPECT/CT, which shows increased perfusion and microtrauma, although access and cost limit clinical use. Aktuğlu and Kayaokay (2019) described “bone bruise” patterns, interpreted as subcortical microfractures, which may be similar to non-inflammatory early CN.
While early-stage X-rays have limited diagnostic value, Rosskopf et al (2019) advocated for their baseline use to monitor potential progression and deformity. PET and scintigraphy also show potential, though practical barriers remain. All authors agree that early MRI should be pursued aggressively, even without full clinical confirmation.
Biomarkers
Biomarkers are generally not reliable indicators of CN. Petrova et al (2007) and Vopat et al (2018) found no consistent elevation of inflammatory markers like CRP, ESR or WBC, especially in early stages. Botek et al (2010) and Maden and Pai (2013) echoed that these are more useful in differentiating active infection than CN.
However, alkaline phosphatase, a liver function test associated with bone turnover, may help monitor disease progression. This is supported by NICE (2019) and has clinical relevance for ongoing management rather than initial diagnosis.
Peripheral vascular disease
Several studies emphasise the role of vascular status in CN presentation. Marano et al (2016) compared diabetic patients with and without CN and found that CN patients had a higher prevalence of moderate to severe arterial calcification and stenosis. This compromised perfusion may affect the inflammatory response, reducing the limb’s ability to show classic CN signs.
Meloni et al (2022) reported that the prevalence of peripheral arterial disease in CN patients ranges from 11% to 40%. As a result, clinicians should consider vascular compromise as a confounding factor in diagnosis. Patients with poor perfusion may not exhibit temperature increases or inflammation even in the presence of active CN.
Conclusion
The consistent patterns in early clinical presentations, as demonstrated in the case studies and supported by the literature, strongly suggest the need to revisit and expand the current Eichenholtz classification system. Specifically, we propose the addition of a non-inflammatory stage preceding stage 0, in which patients report new pain or sensation in a previously insensate, neuropathic foot – without the hallmark signs of inflammation, such as swelling, redness, or elevated skin temperature.
At this early stage, accurate diagnosis relies heavily on thorough clinical assessment. A detailed musculoskeletal examination is essential and should include joint mobilisation techniques (e.g. the piano key test) and direct palpation of foot joints to identify localised tenderness or discomfort. These assessments should be conducted bilaterally to compare findings with the unaffected foot. Notably, any newly reported pain or altered sensation in a neuropathic foot, in the absence of absent inflammation or postural changes, should raise suspicion for a potential non-inflammatory phase of CN.
Vascular assessment remains a critical component, particularly as the presence of peripheral arterial disease may mask or dampen the typical inflammatory response, potentially delaying recognition of early-stage CN. In this context, symptoms may instead reflect early bone marrow oedema and microvascular trauma—physiological changes that may precede the inflammatory phase and could explain reported pain or altered sensation.
While MRI remains the most sensitive imaging modality for both inflammatory and non-inflammatory stages, clinical judgment, careful history-taking, and physical examination become even more important during this subtle, early phase. In cases of diagnostic uncertainty, conservative management with offloading and monitoring may be justified pending further imaging.
There has long been debate regarding the difficulty of diagnosing CN in its earliest phase. This article contributes to that discussion by highlighting the potential existence of a prodromal, non-inflammatory stage and advocating for its inclusion in the formal staging of CN. We propose a revised five-stage model: non-inflammatory, inflammatory, fragmentation, coalescence, and consolidation.
In summary, the clinical findings from the two case studies, along with supporting literature, underscore the need to enhance clinical awareness of this subtle, early phase of CN. Recognising and responding to the non-inflammatory stage could lead to earlier intervention, potentially preventing severe foot deformities, ulceration, and lower-limb amputation. We hope this article serves as a catalyst for further research, clinical validation, and updated guidelines to improve patient outcomes.