Glucocorticoids (or corticosteroids) are widely prescribed in primary care for a variety of conditions. In the previous issue of the Journal, the mechanisms by which glucocorticoids (referred to here as steroids) affect glucose metabolism, and the most high-risk situations in which hyperglycaemia or diabetes might be induced, were described (Morris, 2018). This article focuses on the monitoring and management of steroid-induced diabetes and hyperglycaemia (see Box 1), with an emphasis on blood-glucose-lowering drugs, including insulin.
Glucose monitoring and targets
The Joint British Diabetes Societies’ (JBDS) statement for inpatient care of individuals without prior diagnosis of diabetes receiving oral steroid therapy recommends once-daily capillary blood glucose monitoring for individuals judged to be at high risk of steroid-induced diabetes (Roberts et al, 2018). In the case of a once-daily morning dose of steroid, glucose monitoring is suggested any time between pre-lunch and post-evening meal.
It is important to realise that a fasting blood glucose may underestimate glucocorticoid-induced hyperglycaemia and diabetes because the glycaemic consequences of a morning dose of steroid will be most evident from lunchtime through to the evening, with glucose levels falling overnight (Clore and Thurby-Hay, 2009; Kwon and Hermayer, 2013; Tamez-Perez et al, 2015). This argument holds well for prednisolone, an intermediate-acting glucocorticoid, but for dexamethasone, with its longer half-life, steroid-induced hyperglycaemia will persist longer with less of a decline during overnight fasting (Tamez-Perez et al, 2015).
Translating this advice into a primary care situation, it has pragmatically been suggested that screening should be undertaken once or twice weekly, with a 2-hour post-lunch capillary blood glucose (CBG) test in patients without known diabetes, but who are at risk of steroid-induced diabetes (Mills and Devendrea, 2015). In highest risk situations, this could be increased to daily monitoring. Ideally, an HbA1c at the onset of steroid treatment would help to identify pre-existing undiagnosed type 2 diabetes (or pre-diabetes), but, thereafter, is not useful in monitoring until around 3 months after initiation of steroid treatment (Mills and Devendrea, 2015; Roberts et al, 2018).
If CBG readings are <12 mmol/L, continued monitoring is appropriate as evidence suggests that around half of steroid-induced diabetes arises between the second and fourth week of treatment (Gonzalez-Gonzalez et al, 2013). If CBG is >12 mmol/L, monitoring frequency should increase and venous blood samples should be sent to the laboratory. If venous blood glucose levels are 11.1 mmol/L or more, the diagnosis of steroid-induced diabetes can be made (two samples are required if the person is asymptomatic; Mills and Devendrea, 2015; Tamez-Perez et al, 2015; Roberts et al, 2018).
For those commencing steroids with known type 1 or type 2 diabetes, JBDS recommend monitoring pre-meal and pre-bedtime (i.e. four times daily), with intensification of treatment if blood glucose levels exceed 12 mmol/L (Roberts et al, 2018).
The Joint British Diabetes Societies for Inpatient Care (JBDS-IP) guideline recommends an ideal CBG range between 6 and 10 mmol/L, with 4–12 mmol/L being acceptable (Roberts et al, 2018). Targets do, however, need to be individualised and, if necessary, relaxed to reduce the risk of hypoglycaemia (e.g. in the frail elderly, people with dementia, those with other comorbidities, including cardiovascular and renal disease, or in end-of-life care).
Management of hyperglycaemia secondary to steroid use
As usual, the starting point in the management of individuals with steroid-induced diabetes should be lifestyle education with an emphasis on diet, exercise and weight loss. Those maintained on oral corticosteroids should carry a steroid treatment card (counselling against abrupt withdrawal of treatment). Do not forget to address cardiovascular risk and, where necessary, smoking cessation, treatment of hypertension and use of statins should be instituted to prevent macrovascular complications.
If blood glucose monitoring (BGM) reveals readings persistently above target, then blood-glucose-lowering treatment should be initiated. There is a lack of evidence to guide best treatment pathways, but an important point is to match the glucose-lowering profile of medication to the hyperglycaemic excursions induced by the steroid regimen (Perez et al, 2014).
Treatment should be individualised. When hyperglycaemia is mild and the duration of steroid therapy limited to a week or two, then conservative management may be sufficient. Oral agents may be tried initially with persistent mild hyperglycaemia (Tamez-Perez et al, 2015).
Sulfonylureas and meglitinides
Sulfonylureas, such as gliclazide, glipizide or glimepiride, are the first-choice oral treatments for hyperglycaemia arising from use of steroids as their impact on blood-glucose lowering is immediate and post-prandial hyperglycaemia is improved (Mills and Devendrea, 2015; Roberts et al, 2018). In terms of timing of doses, in the case of a once-daily morning dose of steroid, one option would be to start with gliclazide 40 mg with breakfast and uptitrate the dose in 40-mg stages every 48 hours to a maximum of 240 mg, as guided by CBG readings. These need to be taken on multiple occasions during the day to identify hypo- or hyperglycaemia. (For higher doses of prednisolone, starting and titration doses may be increased.) If this fails to achieve adequate glycaemic control, an evening dose of 80 mg gliclazide could be added (Roberts et al, 2018). Throughout this process, patients will need to be aware of the risk of hypoglycaemia, and how to identify and respond to it.
Mention should be made of the meglitinides (repaglinide and nateglinide) as an alternative to sulfonylureas. Like sulfonylureas, these agents are insulin secretagogues, but have a quicker onset and shorter duration of action. They could be given with lunch and evening meal to more specifically target the post-prandial hyperglycaemia induced by steroids, possibly with a lower risk of hypoglycaemia (Suh and Park, 2017).
Other non-insulin therapies
The insulin sensitisers metformin (provided renal function is satisfactory and gastrointestinal symptoms are tolerated) and pioglitazone (in the absence of heart failure or left-ventricular dysfunction, fracture risk and unexplained haematuria) may be tried, although onset of action is relatively slow (Willi et al, 2002).
The incretin agents, dipeptidyl peptidase-4 (DPP-4) inhibitors (gliptins) and glucagon-like peptide-1 (GLP-1) agonists, act by stimulating insulin secretion and suppressing glucagon production post-prandially. With an inherently low predisposition to hypoglycaemia and quick onset of action, they are potentially well suited to dealing with steroid-induced hyperglycaemia (Yanai et al, 2010; Matsuo et al, 2013; El Ghandour and Azar, 2014).
The mechanism of action of sodium–glucose cotransporter 2 (SGLT2) inhibitors (provided renal function allows) suggests that they could be useful in dealing with hyperglycaemia induced by steroids, but firm evidence of their effectiveness is yet to be gathered (Mills and Devendrea, 2015; Tamez-Perez et al, 2015).
Insulin
If glucose targets are not reached on maximum doses of sulfonylurea (or meglitinide), with or without the use of other antidiabetes agents, then insulin should be considered (Suh and Park, 2017; Roberts et al, 2018). Indeed, if blood glucose levels are particularly high, then insulin may be the best initial option (Tamez-Perez et al, 2015). The use of insulin is increasingly likely the higher the dose and potency of the steroid.
Insulin has an immediate onset of action and uptitration to an effective dose is relatively easy. Clearly, individuals will require education around BGM (multiple times daily) and adjustment of insulin dose as well as recognition and management of hypoglycaemia. Advice on injection technique and sharps disposal, and discussion of insurance and driving regulations is also recommended. At this stage, it is likely that referral to the diabetes specialist team will be necessary.
Human NPH (isophane) insulin administered once daily in the morning is accepted as the insulin of choice because, as an intermediate-acting basal insulin, its activity profile (defined by half-life) matches the hyperglycaemic profile induced by a morning dose of oral steroid (Roberts et al, 2018). Used in this manner, the risk of nocturnal hypoglycaemia is minimised.
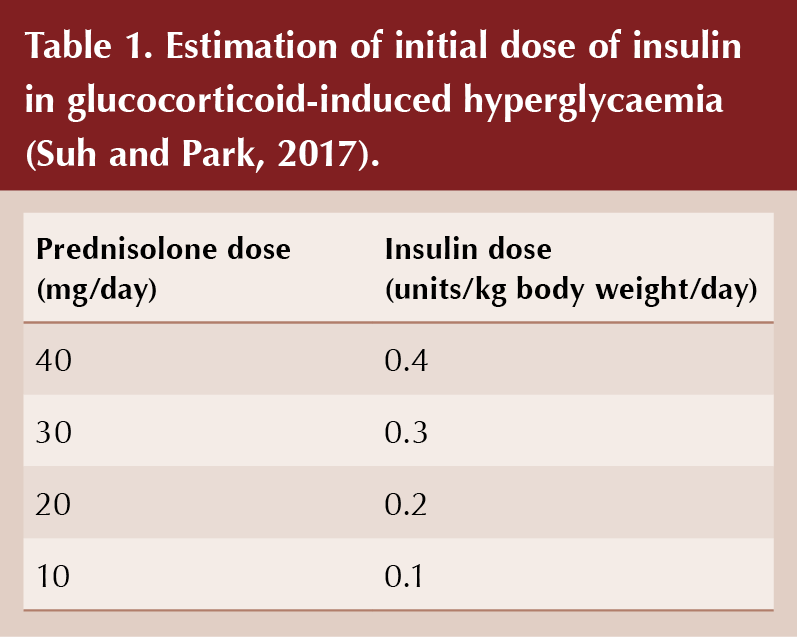
The starting dose of NPH insulin should be proportional to the dose of daily steroid, taking account of body weight (see Table 1; Tamez-Perez et al, 2015; Suh and Park, 2017). Subsequently, the insulin dose can be increased or decreased in 10–20% increments according to CBG readings taken every 24–48 hours. If fasting blood glucose readings are controlled (5–7 mmol/L) but post-prandial readings remain high despite use of NPH insulin, then a rapid-acting insulin analogue could be introduced with lunch and/or evening meal (e.g. insulin aspart, insulin lispro or insulin glulisine).
More complex steroid regimens
If multiple daily doses of steroid are used (e.g. dexamethasone twice daily), then blood glucose levels are likely to be elevated throughout a 24-hour period. Under these circumstances gliclazide should be commenced at 40 mg twice daily (or 80 mg twice daily, depending on steroid dose), titrating up to 160 mg twice daily, as necessary. Twice-daily NPH insulin would be appropriate here or, more simply, a single morning dose of a longer-acting insulin analogue could be chosen (e.g. insulin glargine 100 units/mL, insulin glargine 300 units/mL, insulin detemir or insulin degludec; Tamez-Perez et al, 2015; Roberts et al, 2018).
Pre-existing type 2 diabetes
With pre-existing type 2 diabetes, the use of sulfonylureas is recommended (as described above) for steroid-induced hyperglycaemia if blood glucose levels are above target (Roberts et al, 2018). If a sulfonylurea is already being utilised, it can be titrated up to maximum dose. If this is already the case, other agents may be uptitrated or added to achieve target levels, although their evidence base is limited in this situation. If necessary, insulin may be initiated, as previously described (Roberts et al, 2018).
Diabetes already treated with insulin
If the person with type 2 diabetes is already maintained on a once-daily basal insulin, then, in the case of a once-daily morning oral steroid, this should be switched to a morning injection (if injected in the evening) and titrated upward in increments of 10–20% based on pre-evening meal glucose levels (Roberts et al, 2018). When a basal insulin proves insufficient, the addition of rapid-acting insulin analogues with lunch and evening meal should be considered.
If the individual is already following a more complex insulin regimen, doses can be titrated up, bearing in mind the need to match dose increases with the hyperglycaemic time profile induced by the steroid regimen. Thus, for those taking a twice-daily biphasic insulin for a once-daily morning steroid regimen, increases in the morning dose of insulin would be appropriate. More flexible than this, however, is a basal–bolus regimen (likely to be already in place in type 1 diabetes), which allows increases in rapid-acting insulin with meals to counter the post-prandial rises in blood glucose induced by steroid treatment.
Reducing corticosteroid therapy
In the majority of cases of people taking steroids, the aim will be to reduce and ultimately stop steroid therapy. Bear in mind that whilst individuals receiving high-dose steroids for 2 weeks or less can safely undergo abrupt cessation of therapy, for those taking longer steroid courses the dose must be tapered slowly to counter the risks associated with possible adrenal suppression.
When reducing corticosteroid dose, a corresponding down-titration of doses of insulin and sulfonylurea will be necessary to avoid hypoglycaemia. Careful monitoring of blood-glucose levels during this period is essential to achieve this safely. It may take a few days before the reduction in steroid dose translates into reduced blood-glucose levels. For a weekly 5-mg reduction in prednisolone from a 20-mg daily regimen, a 20–25% reduction in insulin dose, or a 40-mg reduction in gliclazide, is appropriate (Roberts et al, 2018).
Following cessation of steroid therapy (and corresponding reduction in blood-glucose-lowering medication), BGM should continue in anticipation of a return to pre-steroid therapy blood-glucose levels. Importantly, some will have had unrecognised type 2 diabetes prior to steroid treatment and will need continued treatment for hyperglycaemia. An HbA1c test 3 months after steroid therapy should be performed as a definitive test for diabetes status (Roberts et al, 2018).
The question arises as to whether diabetes has resolved when levels of normoglycaemia return in individuals with steroid-induced diabetes who are subsequently not taking any pharmacological treatment for hyperglycaemia. However, even when normoglycaemia is achieved, there remains the risk of emergence of retinopathy and other microvascular complications (Hillson, 2017). Thus, it has been recommended that the appropriate coding is “Diabetes in remission”, to ensure that these people remain under surveillance and are still invited for screening for diabetes complications (Hammond, 2016).
Steroid treatment in end-of-life care
Corticosteroids are a frequently used treatment in palliative care for a variety of diagnoses and can provide symptomatic relief of nausea, anorexia, weight loss and fatigue (Pilkey et al, 2012). High-dose dexamethasone is commonly used with primary or metastatic brain tumours and the risk of steroid-induced diabetes or worsening of hyperglycaemia in existing diabetes is particularly high in this situation. However, Pilkey’s review found that even in people not taking high-dose oral steroids, there was a significant risk of diabetes.
Again, in the absence of a robust evidence base, management has tended to be based on experience (Quinn et al, 2006). The importance of a holistic approach has been emphasised (James, 2013; Diabetes UK, 2018).
Capillary blood glucose readings in the range 6–15 mmol/L have been suggested as a target in end-of-life care (Diabetes UK, 2018), although individual circumstances must always be prioritised and this will reflect life-expectancy. The aim is to avoid symptomatic hyperglycaemia (including the possibilities of diabetic ketoacidosis [DKA] or hyperosmolar hyperglycaemic state [HHS]), whilst minimising the risk of hypoglycaemia. The frequency of monitoring for new onset of diabetes should be agreed with the individual or their advocate.
Treatment strategies are similar to those outlined earlier in this article and algorithms for steroid-induced hyperglycaemia are included in Diabetes UK’s End of Life Diabetes Care document (Diabetes UK, 2018).





Jane Diggle discusses emotional health and diabetes distress, and offers some tips for discussing this in our consultations.
11 Nov 2025